Hybrid eConsent for on-site or remote patient enrollment
Create an effortless experience for your patients with an all-in-one experience to remotely recruit, screen and enroll patients from the comfort of their home.
Schedule demo
Castor’s all-in-one eConsent platform
Castor’s suite of modular eConsent solutions creates clinical trial efficiencies from recruitment and screening, all the way to direct data capture and analysis. Each virtual element can be integrated into your traditional, hybrid, or fully decentralized trial as a standalone solution, or packaged together as an all-in-one eConsent platform.

Recruit and enroll participants with a seamless onboarding experience
Increase patient access and diversity with remote enrollment. Recruit your participants through a customized recruitment portal and pre-screen with questionnaires to help filter appropriate participants. After consent, patient data from enrolled participants can automatically be updated in Castor EDC, ready for the next step.
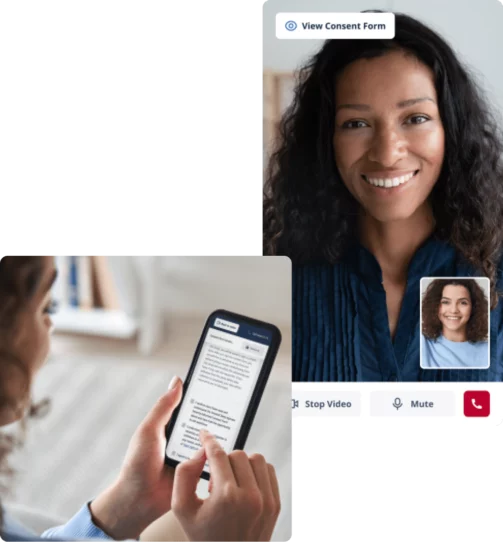
Consent study participants remotely with secure video visits
Host remote consent visits with video and engage participants in the comfort of their home. No switching between applications – video calls, signatures, and questionnaires are all built-in to reduce complexity and increase your patient’s experience.
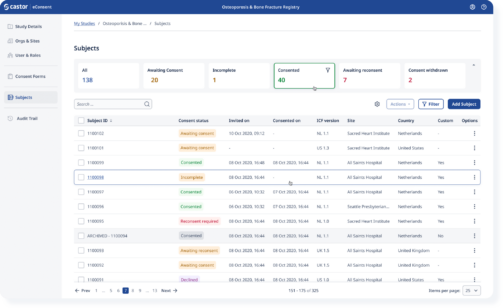
Easily track enrollment progress and gain real-time insights
Study coordinators can see in real-time where patients are in their enrollment process and assist patients while meeting regulatory requirements.
17,824 patients in 15 weeks
Launching a DCT on Castor’s platform
World’s first study to use machine learning in diagnosis of COVID-19, supported by TAKEDA and Julius Clinical

“Using Castor, we succeeded in recruiting over 17,824 patients enrolled in 15 weeks during a global pandemic, an achievement that could not have been accomplished without tools purpose-built with decentralized trials in mind.”
Marcel Van Willigen
Julius Clinical
#0
Ranked EDC
0 K +
Happy Users
0 K +
Studies
0 %
Customer Satisfaction
0 +
Countries
0 M +
Patients
0 %
On time delivery
Three ways to get started
Key features

Video visits for remote eConsent
No 3rd party plugin or download required. Ensure transparency and enhance study comprehension for study subjects with secure, encrypted video calling.
Automated and digital patient recruitment
Study subjects can enroll themselves organically using a customizable patient portal landing page and/or study managers can manually send patients invites to enroll in a clinical trial.
Versioning & ICF management
Multiple consent forms are available for a single site and/or across multiple sites. ICF statuses and versioning ensures sites are always using the correct version. New ICFs can be created quickly by duplicating existing ones. Patients re-consent for amended protocols.
Compliance
21 CFR Part 11 electronic signature compliant and industry leading encryption, protects patient privacy, and ensures tamper-proof eConsent. Full audit trail and multi-factor authentication ensure ease of use and security.
Integrated platform and open architecture
Native integration with Castor product suite (EDC and ePRO app) to allow uninterrupted data capture flow in a single ecosystem. Flexibility to integrate with other eClinical technology vendors through RESTful API.
User management
Flexible roles that help the consent workflow and multicenter study responsibilities: Patients, Care-givers/Guardians, Study Admin, Site Admin, Investigator, Monitor and Read-only users.
Featured resource
eConsent readiness in 2024
Download this resource to learn how eConsent is managed and implemented in the top countries, for clinical research.
Castor’s electronic informed consent software accelerates enrollment & increases retention
We are trusted by


