Electronic Data Capture (EDC)
Castor’s electronic data capture (EDC) makes it easier to capture your trial data and integrate it seamlessly with other data in your clinical trial ecosystem. Castor ranks among the top 5% of EDC’s for shortest build time, with more than 90% of our studies being deployed within the first 4 weeks.
Schedule demo
Capture and re-use research data from anywhere, anytime.
Explore Castor EDC/CDMS, our most robust module, and how it can help you capture and manage all your study data in one centralized hub. And if your needs go beyond, easily layer in eConsent and ePRO to build a full ecosystem of research tools.
Create better data flow
Connect and manage all trial components and integrations, in one place.
- Integrate data from EHR, eCRF, ePRO/eCOA, laboratory, wearables, and other devices.
- Work with any software or database in your clinical trial ecosystem with Castor’s open API.
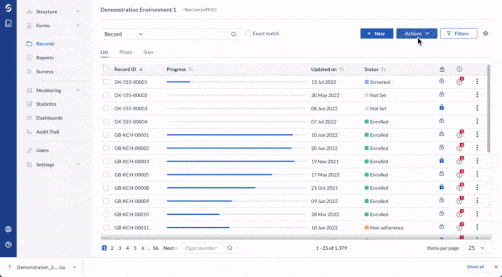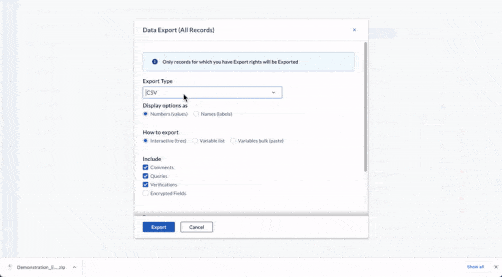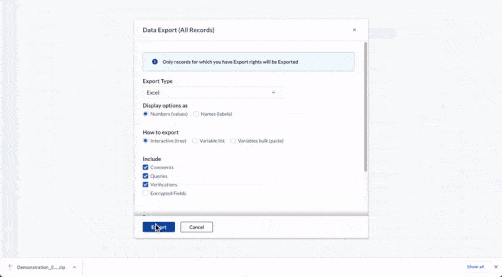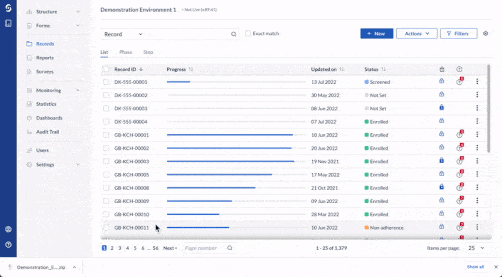

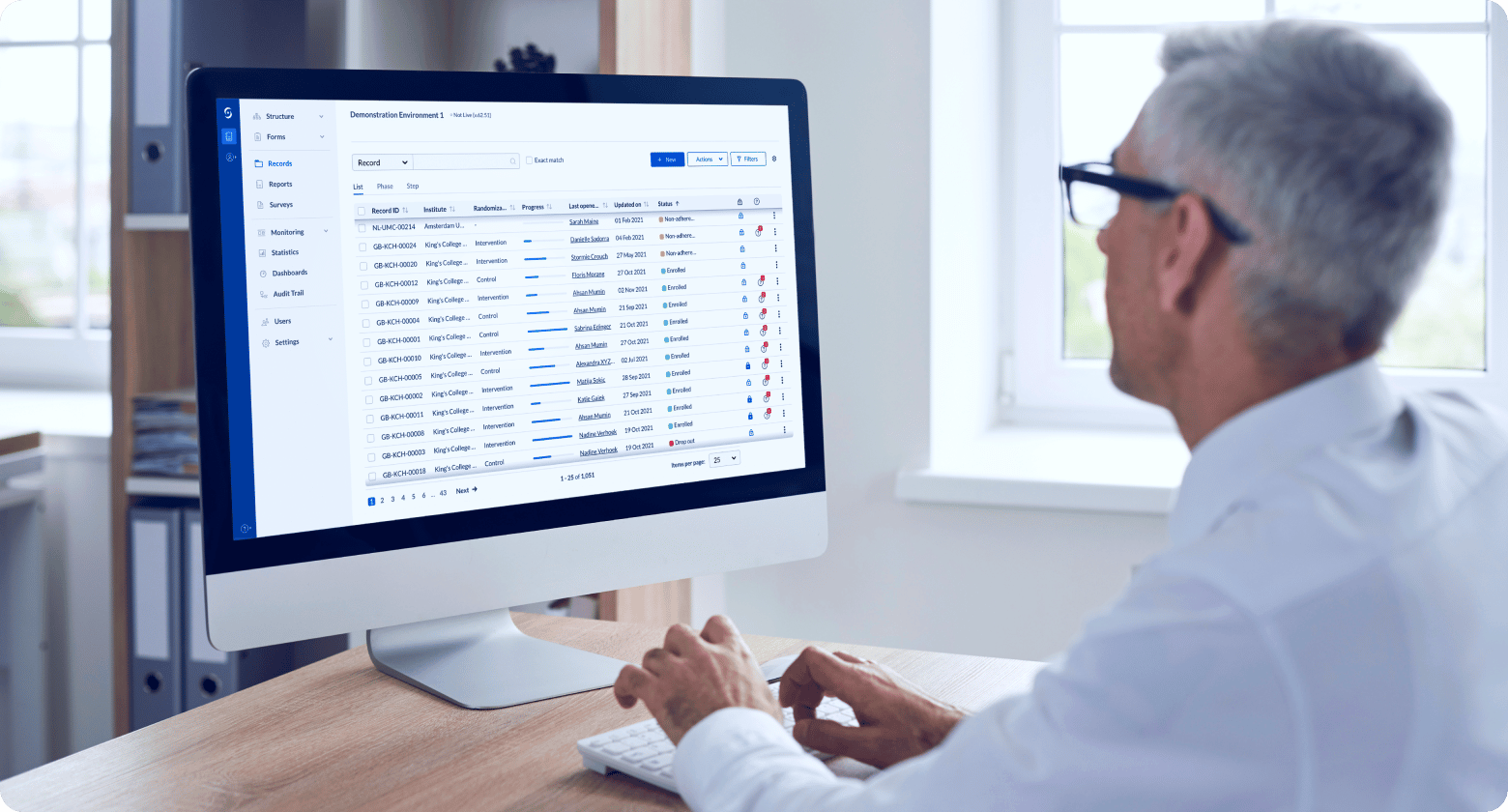
Gain real-time visibility
Monitor study progress and outcomes in real-time with Castor’s study health dashboard.
- Get an overview of study statistics as your studies are ongoing.
- Track record data entry progress and outstanding queries.
- View Verification Status (SDV) for Steps.
Generate compliant data, confidently

Build advanced eCRFs in minutes
- Start with one of our pre-built eCRF templates.
- Customize your form with 21 different field types.
- Clone and re-use forms as you build more studies.
Store data securely
- Save study data in real-time.
- Store it automatically on certified, compliant servers in any country.
- Protect your data with 25-year data retention, field-level encryption, and two-factor authentication.
Amend studies with less risk
- Simplify protocol amendments by using a tool that’s secure, trackable, and easy-to-validate.
- Easily create test environments for each of your subsequent studies.
Achieve global compliance
- Meet compliance certifications worldwide such as FDA CFR Part 11, GDPR (EU), ICH GCP (HIPAA, US), ISO 27001, and ISO 9001.
- Align with GCP, HL7 FHIR, and other regulatory guidelines.
Build fully-integrated workflows
“It was easy to create our clinical trial workflow. Integration and training with external partners was effortless. Usability and light coding were essential to us. We were able to test and build our own studies with minimal support and admin.”

Arne Böhling
Clinical Affairs Director, Essity
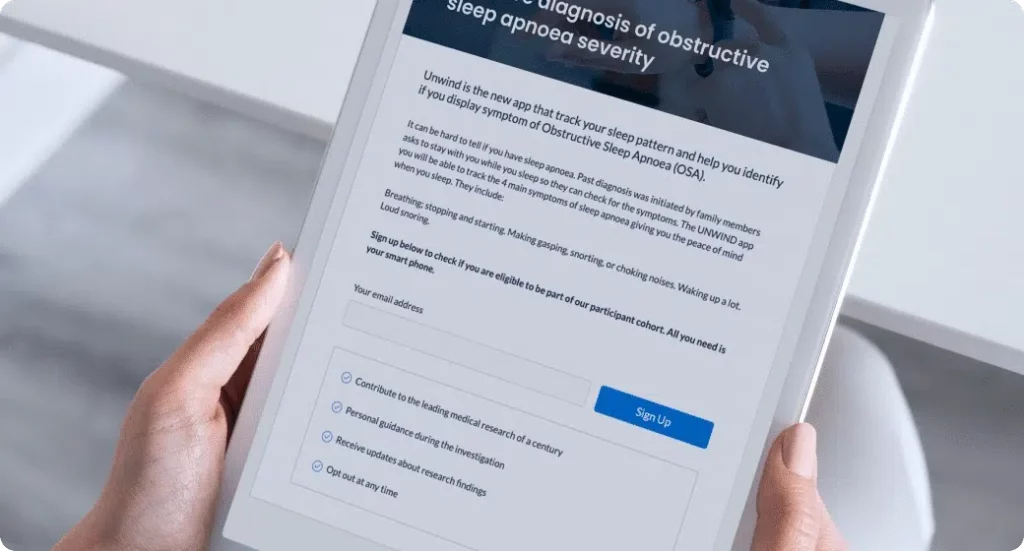
Recruit, screen and consent
Participants are onboarded remotely via dedicated landing pages, pre-screening, and video-enabled informed consent.

EDC & eSource platform
Connect all your DCT components and support direct data capture in the field.
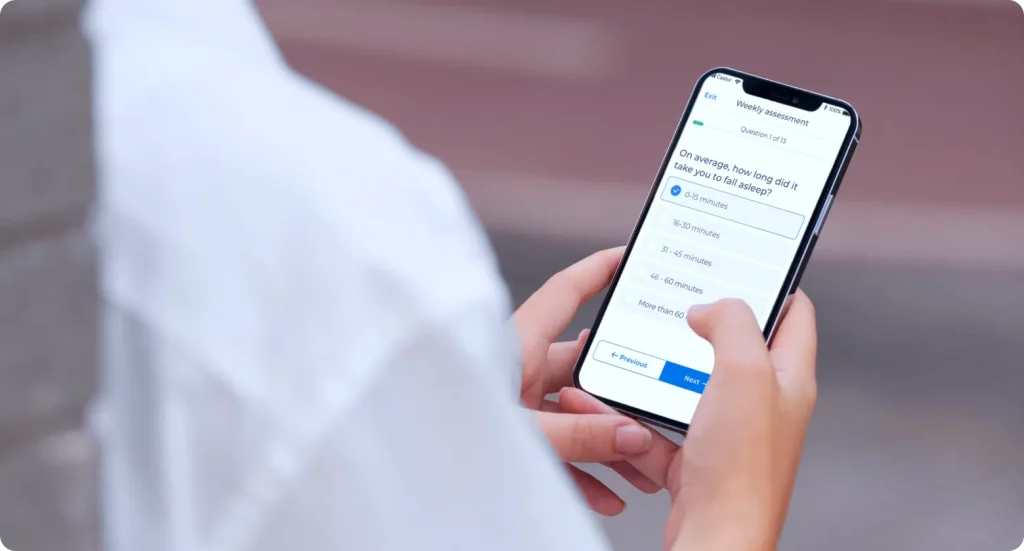
ePRO & eCOA
Put your patient diaries and surveys on a user-friendly mobile app that connects your participants from the comfort of their home.
Ready to get started?
#0
Ranked EDC
0 K +
Happy Users
0 K +
Studies
0 %
Customer Satisfaction
0 +
Countries
0 M +
Patients
0 %
On time delivery
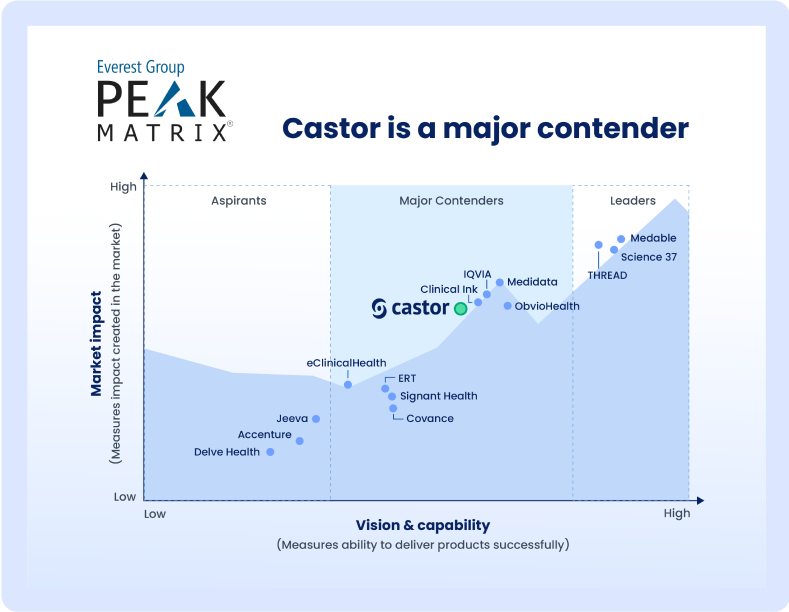
Analysts respect us
Clinical trials succeed when data flows. That’s why Castor is reengineering traditionally stodgy processes with easy-to-use digital tools that remove barriers for participants and researchers.
See why our company was chosen as a Major Contender impacting the market in the Everest Group’s PEAK Matrix® of Life Sciences Electronic Data Capture Products Assessment 2024.

Featured resource
eCRF in clinical trials: Shifting to a modern research paradigm
Explore how we can bridge the gap from siloed, cumbersome clinical trial processes to fully connected, interoperable clinical trial platforms for collecting and analyzing data.
Start your eClinical journey with Castor’s EDC
Over 500 trusted partners