What makes Castor EDC a better and more efficient research tool? As a researcher, are you using general tools like Microsoft Excel, Microsoft Access, Google Forms or SPSS to capture data for your research?
Castor EDC works for you whether you’re building a small departmental observational study, a registry or a multicenter Randomized Controlled Trial (RCT).
Continue reading to find out The Good and The Bad of these other tools. Or scroll straight down to one of the seven reasons why we believe Castor is the better choice, because these tools:
1. Are not meant for medical data capture
2. Are not designed with researchers in mind
3. Have no dedicated online resources for medical research
4. Have no one to ask if you get stuck
5. Are neither secure nor compliant
6. Do not support multicenter trials
7. Do not support randomization

Common EDC Alternatives
- Microsoft Excel is a spreadsheet application which many researchers resort to for electronic data capture.
- Microsoft Access is also used by some people a database management system which combines a database engine with a graphical user interface and software-development tools.
- Google Forms is often used for creating and sending out research surveys directly to patients.
- SPSS stands for Statistical Package for the Social Science and is one of the most commonly used statistical packages within the scientific research community. SPSS is meant for complex analysis and manipulation of data, but is often used by researchers to capture research data as well.
The Good
Familiar for most. Most researchers are familiar with Excel, Access, Google Forms and SPSS and have used them at one time or another.
No new training necessary. Most researchers do not need any training for using these general-purpose tools due to previous experience using them. Even otherwise, these applications are easy enough to master on your own.
Import and export of data is easy. Both Excel and Access allow you to import or link directly to data stored in other applications and other databases.
Freely available to researchers. Most universities provide researchers with Excel and Access free of charge, in some cases an additional fee must be paid for SPSS. They are likely to be already present on your desktop.
Google Forms enables easy information collection from patients. Google Forms is an easy option when collecting information directly from patients, since it can be sent out as a survey. Google Forms also makes it easy to collaborate on eCRF design with other researchers.
The Bad
1. Not meant for medical data capture
Excel is a spreadsheet application. Access is a database management system. SPSS is primarily a statistical analysis application. None of these were created for the purpose of electronic data capture for medical research.
For example, although Excel and Access have data input validation features, they are cumbersome and complicated to set up.
Castor EDC has been specifically designed for medical research. It solves many problems medical researchers face when using multi-purpose tools for EDC.
- Easy drop down menus to set field validations and limits in form design enhance your data input accuracy.
- No programming knowledge required which means you not need to be an IT geek to build your eCRFs. Castor EDC’s Form Builder makes it easy for you to set field dependencies and perform advanced calculations without any prior experience.
- Standardization features incorporated into Castor EDC ensure you can reuse research data, by using metadata to code your fields.
2. Not designed with researchers in mind
They do not offer special guidance for setting up scientific studies and research projects, and this is only to be expected. And for many scientific researchers this can present a challenge.
At Castor EDC, we have changed that. Castor EDC is user friendly because it has been designed for researchers by researchers. We provide you with easy step by step guidance on how to set up your study. With this web application, you can set up your study in a couple of days. Before you design your eCRFs or begin data capture activities, you move through a series of guided steps to set up your study and integrate your study protocols and code book into a new study on Castor EDC.
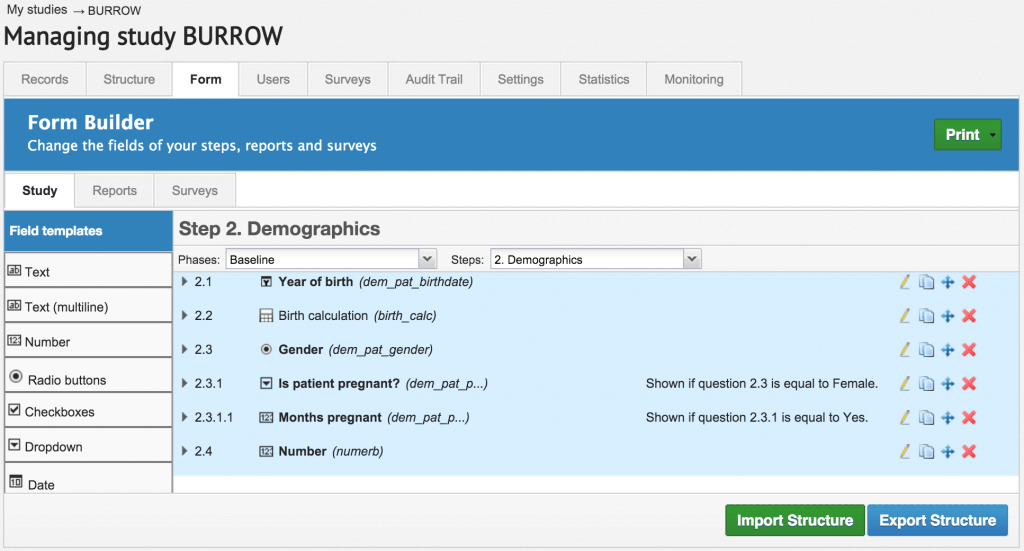
The Form Builder.
3. No dedicated online resources for medical research
Dedicated guides and manuals on how to set up your study in Excel, Access, Google Forms or SPSS are limited or nonexistent. You might find a forum somewhere on the Internet in which your specific problem has been discussed. However, finding information on advanced dependencies and validations might require you to Google quite a bit, spending time you cannot afford.
Also there is no guarantee you will find the information specific to your study in the end. Basically you are on your own when it comes to solving complex problems associated with data validation, randomization and other issues that matter to scientific researchers.
These tools also do not offer guidance on eCRF design. Excel, Access and Google Forms do not provide value added features, templates or productivity tips. As a result, many PhD researchers spend a lot of their precious time—which they could better spend on actual science—in form design using various applications, formatting worksheets and other IT tasks.
You should not be wasting your time this way.
In contrast, Castor EDC offers a wealth of online resources to guide you every step of the way.
- A one-hour online workshop on all the Castor basics to empower you to create your own forms with ease.
- Our Form Exchange allows you to exchange calculations and forms with other researchers using our application. Download your SF-36 or EQ-5D and plug it into your study.
- The Castor Calculation Helper will help you get your advanced risk score calculations up and running in no time.
- Our online manual allows you to quickly find help on any topic.
The Castor EDC online video workshop.
4. No one to ask if you get stuck
Besides Google, who do you ask if you get stuck in Excel, Access, Google Forms or SPSS? Sometimes universities have some support for SPSS, but it might take a long time before you receive an answer.
On average, our helpdesk responds to your queries within one hour via [email protected]
Feel free to describe your technical challenges to us and we will see how we can help. We guarantee an answer to your query within 24 hours on weekdays regardless of your time zone.
And it is not just because it is good for business either. We know we can learn from you.
We take your time and your problems seriously. We want every researcher to have access to a professional EDC, not only those working in sponsored trials or pharmaceutical companies.
To us, helping medical researchers is just part of our job, not a burden. Since all of our customers are medical researchers, your problems and issues have probably been highlighted before.
5. Neither secure nor compliant
Because they were not developed with medical research in mind, these general purpose applications are unsecure and non-compliant with legislation. With regulations tightening across the world, non-compliance with, for example, Good Clinical Practice (GCP) is a problem for all researchers involved in clinical trials.
For example, when you use Google Forms, you do not know where your data is being stored. That is a breach of national and European regulations for conducting medical research.
Castor EDC complies with all applicable laws and regulations for your clinical trials: GCP, 21 CFR Part 11, EU Annex 11, and the General Data Protection Regulation (GDPR). We have been explicitly validated by a large number of hospitals, research institutes and Contract Research Organizations (CROs) that use our product.
Castor EDC also includes an audit trail, that logs every action taken in the system, in line with GCP guidelines.
You can choose in which of our data centers you want to store your information, and we will ensure it never leaves there without your permission!
Find out more on our GCP page and Security Statement how our audit trail, reason for change functionality and secure data storage help keep your data safe and compliant.

Castor’s Dutch datacenter.
6. No support for multicenter trials
Sharing Excel, Access and SPSS files up and down is messy. More importantly, it can compromise data quality and accuracy. It can also easily lead to privacy violations and data security breaches.
Excel and Access allow for assigning authorization levels and locking of different parts of the worksheet or database. But these tend to be complicated. Also, lacking proper rights management means unlocking a worksheet or database can cause all data to become accessible to everyone.
Google Forms allows for collaboration but is not equipped to handle rights management necessary to ensure data is only disclosed to authorized persons.
In contrast, Castor EDC supports everything you need for a multicenter, complex trial. We have built-in features that make it suitable for multicenter RCTs and other studies requiring collaboration and advanced rights management.
-
- Each record in Castor belongs to an institute (center). Authorizations can be set up in minute detail so members of your study team can only access the data they are allowed to access. You can assign authority separately for each user to Add, View, Edit, Delete, Lock, Export, Query, Randomize or Sign eCRFs or parts of them.
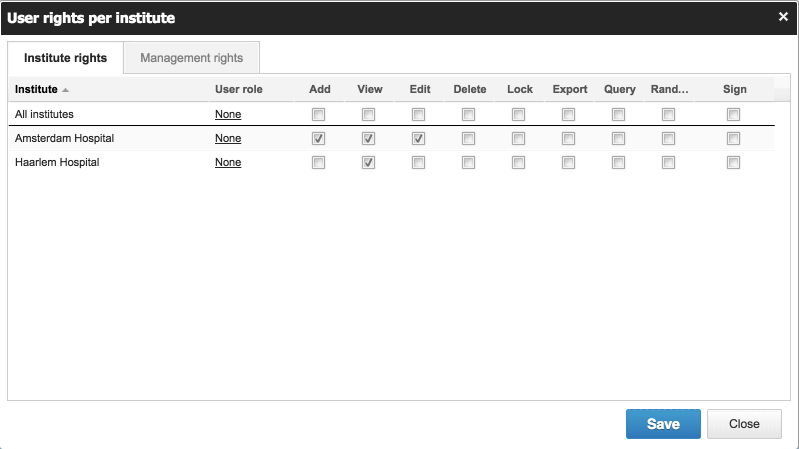
Each Record in Castor belongs to an Institute.
-
- You can also restrict management rights to manage records, forms, users and settings, as appropriate.If you are involved in many different studies, they will show up as a list when you log in to Castor.
- If you are involved in many different studies, they will show up as a list when you log in to Castor.
7. No support for randomization
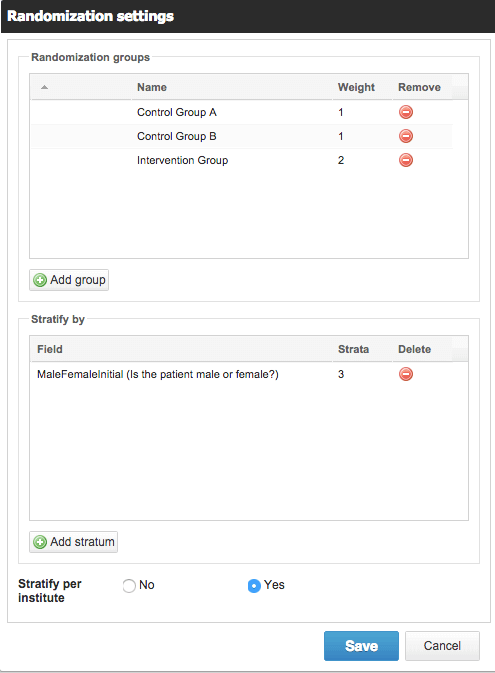
Setting up randomization in Castor is easy.
If you are running a Randomized Controlled Trial (RCT) you will want to carefully organize how you randomize your patients to ensure your study is being carried out in line with your protocol. None of the alternatives considered here have randomization built in. So you must resort to an external tool and feed the results back into your database to randomize effectively.
In Castor EDC, you can use a variable block randomization method that only authorized study team members can see the results of.
