Set your IIS program up for success by giving PIs access to the top-rated EDC system
Ensure data quality and compliance
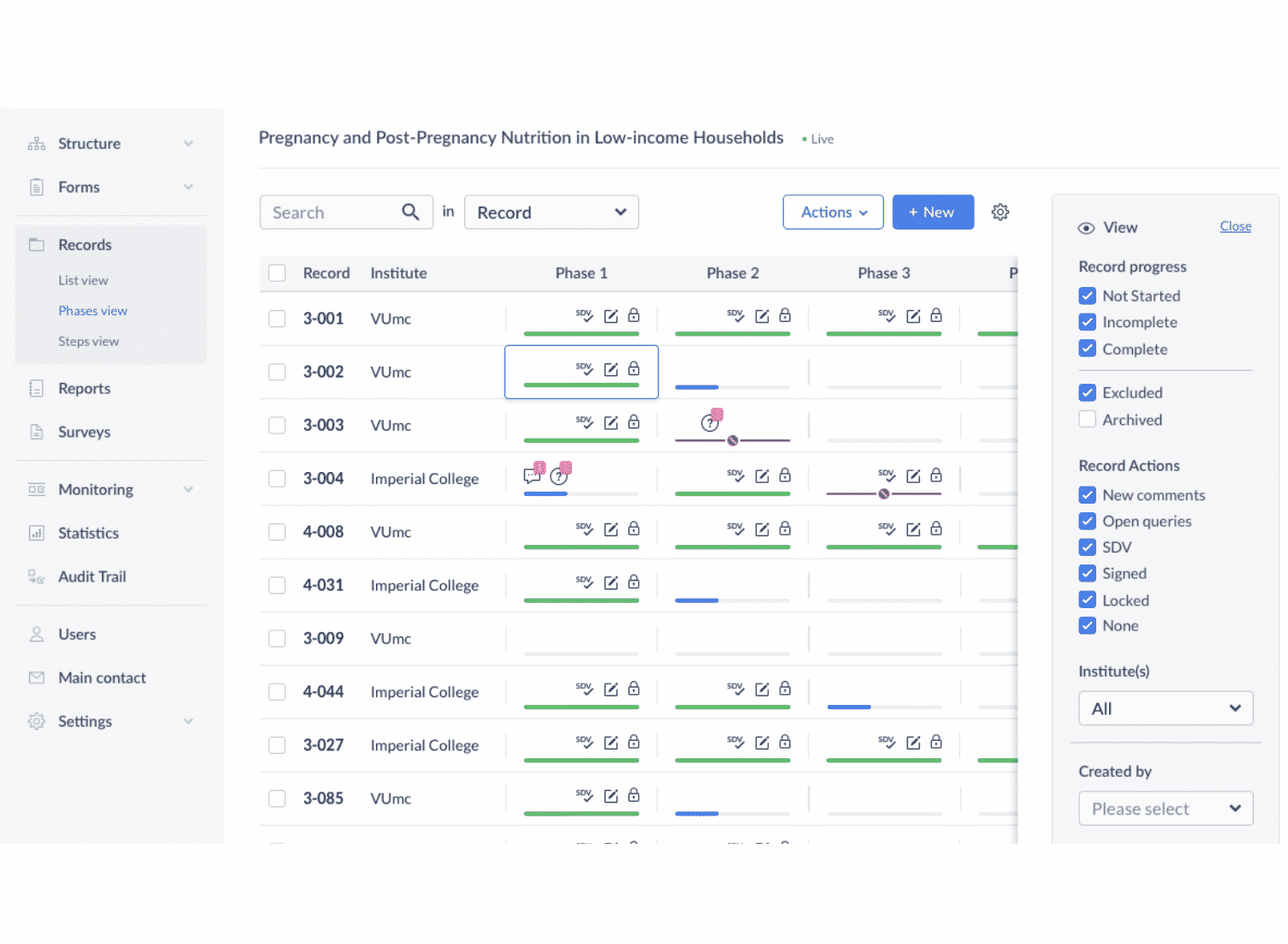
Castor EDC is a commercial-grade data capture system that streamlines the clinical trial process and increases data quality. Study level monitoring capabilities enable PIs to manage data quality and study progression. Castor helps ensure compliance with ISO-14155 and other regulations, and our world-class support team aids investigators from beginning to end.
Learn More
Track IIS progress
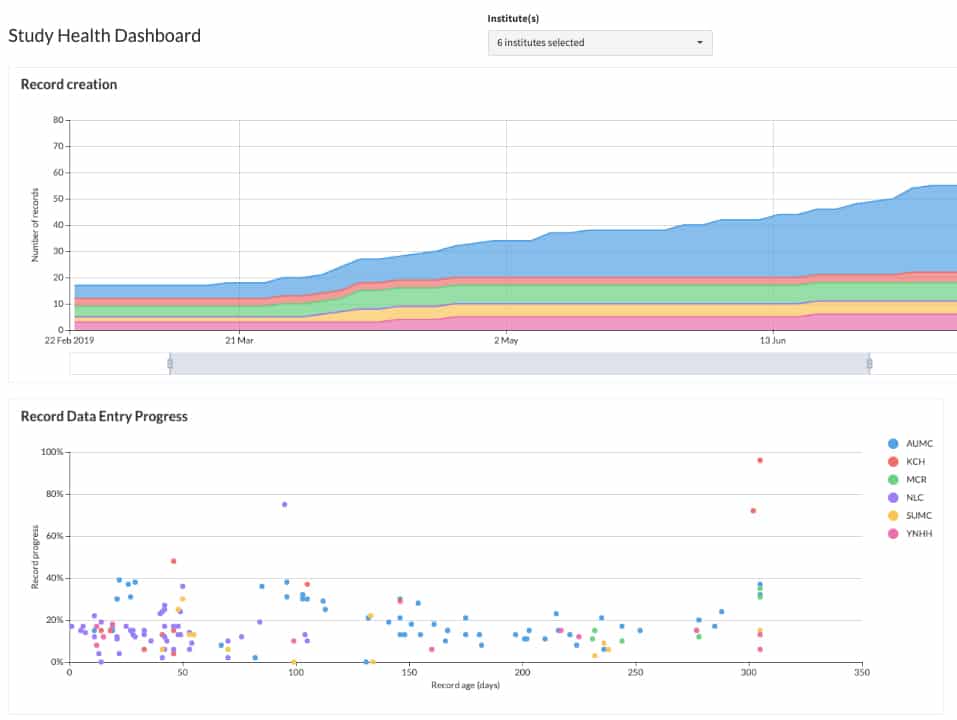
Easily track progress of studies across your IIS portfolio. Castor dashboard provides an overview of the current studies. Drill down—in just a few clicks—to aggregated, real-time data that will answer progress questions without interfering with the study.
Learn MoreMaximize IIS ROI
Aggregated performance, safety, and efficacy data can be repurposed for a variety of uses, including regulatory compliance or marketing projects. Castor ensures completely anonymous transfer of data to medical device companies.
Learn MoreEnsure data quality and compliance
Commercial-grade EDC system for investigators
Commercial-grade EDC system for investigators
- Enable investigators to capture high-quality data and streamline the clinical trial process
- Advanced eCRFs can be built in minutes
- Data can be easily integrated from clinicians, patients, devices, EHR systems
- Advanced and flexible user management
- Study level monitoring to manage data quality and study progression
- Real-time validation checks
World-class support for PIs
World-class support for PIs
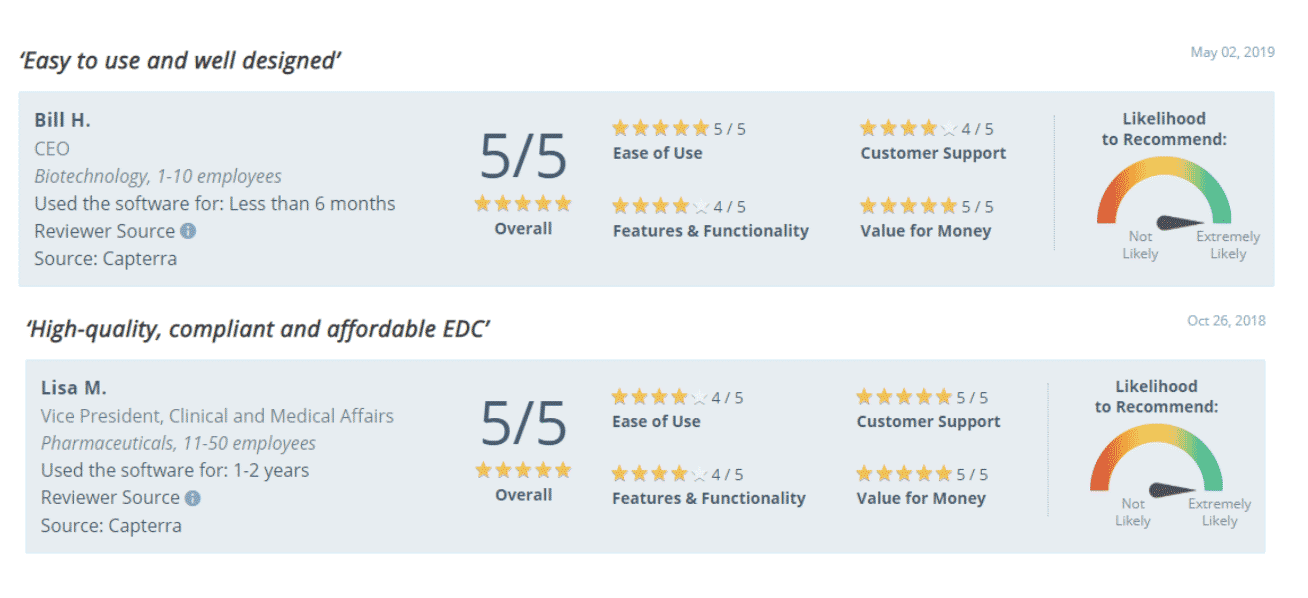
- Consistently high customer approval ratings: 4.7 out of 5 points
- Experts available to assist with study design and setup
- Ongoing support to the central study team and sites
Out-of-the-box compliance
Out-of-the-box compliance
- Compliant with all relevant regulations including GCP and 21 CFR Part 11
- ISO 27001 and ISO 9001 certified
- Enable investigators to perform ISO-14155 compliant data management
- HIPAA-compliant servers in the US
- Supports GDPR compliant studies at European sites
- Global servers enable compliance with local privacy and security regulations
Track IIS progress in real-time
Real-time insights on study progress
Real-time insights on study progress
- Real-time, aggregated insights into study progress
- Overview of all IIS studies provided in single dashboard
- Answer progress questions without interfering with the study
Maximize IIS ROI
Access safety and performance data
Access safety and performance data
- Castor works with investigators to capture critical safety and performance data
- Castor can set up aggregated anonymous reports based on those data points
- Reports can be accessed and securely downloaded by the funder