The 2020 COVID-19 pandemic is one of most transformative events in recent history, affecting nearly every aspect of everyday life. Not only are commerce, education, and millions of lives affected, but the lifesaving research urgently needed to combat the disease also faces serious impediment due to the nature of the crisis:
- How can a medical center continue to operate as a research site while overwhelmed with the critically-ill?
- How can long-term efficacy be assessed in a patient population that is declining over time in most areas?
- How can the varying availability of medications across the world be accounted for to maintain data quality?
To combat the largest pandemic in recent history and generate sufficient evidence of the efficacy and safety of potential treatments, the World Health Organization (WHO) needed to mount an equally massive clinical trial, while at the same time addressing these overwhelming research challenges.
-
Industry
Healthcare
-
Challenge
The WHO needed a user-friendly system capable of being deployed globally, and one advanced enough to support adaptive medication allocation and real-time reporting across many participating countries.
-
Solution
Castor quickly implemented a CRF in six languages, developed an adaptive randomization algorithm fitting WHO specifications and provided 24/7 support.
-
Result
The Solidarity trial has enrolled over 12,000 patients, involved over 2,100 investigators, and activated 553 sites across 30 countries. The trial has eliminated several investigative therapies, allowing for new contenders to be added.
Research Overview
The WHO Solidarity Trial is the world’s largest randomized trial aiming to identify how existing medications might improve outcomes compared to standard of care alone. With more than 12,000 currently enrolled patients out of a planned 15,000, 2,145 investigators, and 553 sites across 30 countries currently participating, efficiently capturing and centralizing this data is critical.
According to the New England Journal of Medicine, “Launching and executing this ongoing trial is a remarkable achievement. The rapid, widespread enrollment is a testament to the commitment of the WHO and its partners [including Castor] and was enabled by a design that allowed, for each patient, the rapid collection, recording, and transmission of a small amount of high-information data. In 6 months, the trial team accumulated information on the collective experience of more than 11,000 hospitalized patients in settings with varied and evolving standards of care, capacity to administer treatment, and treatment options.”
Study Design and Methodology
Adults recently hospitalized with confirmed COVID-19 are randomly allocated between local standard of care, or local standard of care plus Remdesivir, Lopinavir with Ritonavir, or Lopinavir with Ritonavir plus Interferon beta-1a. Originally, hydroxychloroquine was included as an investigational therapy, but was permanently discontinued, as the WHO found it “does not result in the reduction of mortality of hospitalised COVID-19 patients, when compared with standard of care.” Lopinavir was likewise discontinued for futility on June 18, and Interferon on October 16.
The trial follows an adaptive design by necessity, as medication availability will vary by location, and as the WHO’s NEJM publication reported, additional therapies including monoclonal antibodies may be added. Severity of illness at entry is determined by recording if the patient has shortness of breath, has major imaging abnormalities of the lungs, is being given oxygen, or is already on a ventilator.
The primary outcome of the trial is all-cause mortality divided by severity of disease, while secondary outcomes include duration of hospital stay and time to ventilation.
How the WHO Is Using Castor
The WHO needed a user-friendly system that could be deployed globally with 24/7 support, that was advanced enough to support adaptive medication allocation and real-time reporting. The WHO approached Castor to help solve these challenges and quickly selected Castor as its sole EDC provider for the trial.
Castor needed to rapidly adapt and create new solutions to address a number of challenges associated with a trial of this size under the present conditions:
- Castor implemented a CRF in six languages.
- In less than a week’s time, Castor developed and implemented the adaptive randomization algorithm from WHO specifications, which accounted for medication availability by site.
- The constantly-growing number of investigators necessitated an efficient way to add new users. Castor is providing user management and automatically bulk-adds new users every day.
- A QR code field was created to allow investigators to securely connect their mobile devices to the study to upload consent forms, removing the need for handling paper.
- Castor is providing 24/7 support to all Solidarity trial users of its platform.
According to the published paper, “The protocol was designed to involve hundreds of potentially over-stressed hospitals in dozens of countries,” making rapid and user-friendly data capture essential. “Online randomization of consented patients [via Castor] took just a few minutes, as did online reporting of death in hospital or discharge alive.”
By enabling the enrollment and data collection from diverse populations around the world Castor is helping collect data that better reflects real-world efficacy, an essential part of quickly developing treatments for a disease that has claimed more than 1.3 million lives to date.
“In the race against time to identify effective COVID-19 treatments and vaccines, this trial can ultimately enable the world to reduce the number of patients admitted to ICUs and dying from this disease.“
Castor CEO Derk Arts, MD, PhD
“The Solidarity trial is continuing the search for treatments that can modify the progression of COVID-19 and therefore, provide additional means to flatten the curve, while the search for the best vaccine is still ongoing,” said Castor CEO Derk Arts, MD, PhD. “In the race against time to identify effective COVID-19 treatments and vaccines, this trial can ultimately enable the world to reduce the number of patients admitted to ICUs and dying from this disease.“
The ongoing nature of the study reserves the possibility of adding more trial therapies as others are eliminated for lack of efficacy. “So far, only corticosteroids have proven effective against severe and critical COVID-19,” the WHO reports. While the current investigative therapies were not associated with solid evidence of increased mortality, “there were, however, some associated safety signals in the clinical laboratory findings of the add-on Discovery trial.” These results only increase the importance of further researching the safety and efficacy of existing therapies as well as developing new ones.
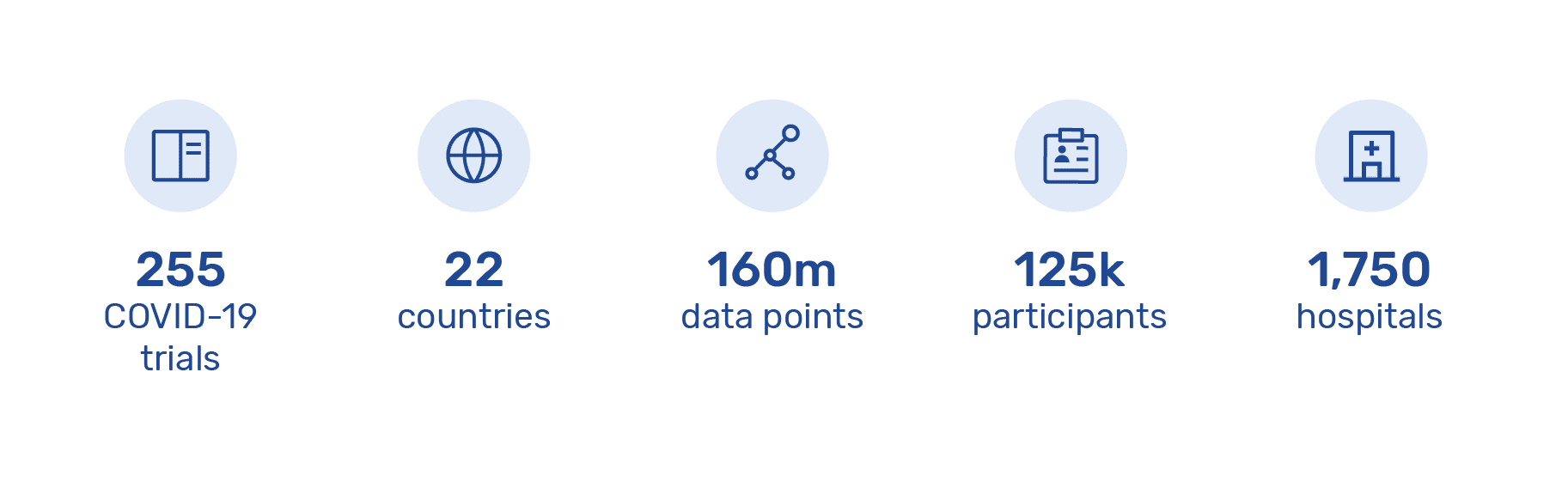
In addition to the WHO trial, Castor is also powering 255 additional COVID-19 trials in 22 countries across 1,750 hospitals. Over 125,500 participants are enrolled in these trials and more than 160,000,000 data points have been captured. By ensuring researchers capture standardized data on Castor’s platform, research data from around the world can be easily aggregated, accelerating the work of researchers developing critical medicines.