To ensure ongoing regulatory compliance under the EU MDR, medical device manufacturers must demonstrate the safety and performance of marketed devices with data obtained through Post-Market Clinical Follow-up (PMCF) studies, which often include patient and physician surveys.
When your PMCF plans include surveying physicians and other healthcare professionals or administering questionnaires to patients, physician survey forms or electronic Patient Reported Outcome (ePRO) forms must be created to prepare for PMCF execution. The Regulatory Affairs Professionals Society (RAPS) described PMCF planning under EU MDR as a burden to manufacturers in an article dated 12 September 2019. To ease this burden, Castor’s technology can help automate PMCF execution so that the entire process can run smoothly and efficiently.
Physician Surveys
Depending on the type of device under PMCF scrutiny, electronic Physician Surveys may be the most effective route to submit relevant PMCF data on medical devices. This might be the case with devices used during certain surgical or endovascular procedures, or with devices that are implanted and require follow-up assessments with a healthcare provider (HCP). In such cases, HCPs may be invited to answer surveys focused on device clinical performance and safety outcomes in either a prospective or retrospective fashion using Castor-generated Clinician Reported Outcome (ClinRO) or Performance Outcome (PerfO) forms.
ePROs
Certain devices may benefit from a different approach in PMCF data collection. For example, devices intended for patient use such as insulin pumps or wearable devices may be better suited for ePRO or Observer Reported Outcome (ObsRO) forms, where patients themselves or their guardians submit PMCF data either prospectively or retrospectively. In such cases, patients/guardians can be recruited through marketing campaigns or via their providers to answer PMCF-tailored questionnaires in the form of ePROs/ObsROs.
How do you create Physician Surveys and ePROs?
With Castor, you can easily set up Physician Surveys or ePROs and send them to survey participants via email in a few, simple steps.
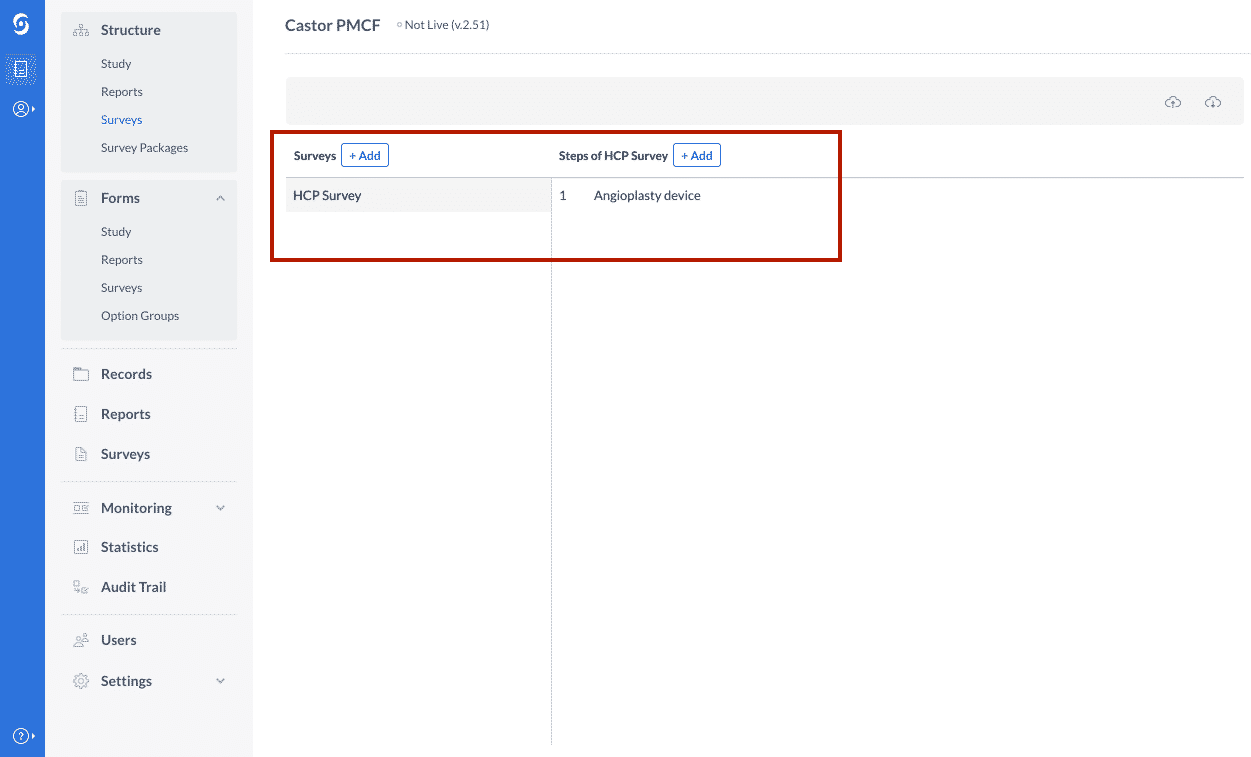
1. First, you will determine the structure of the survey by choosing the survey type as either a Physician Survey or an ePRO and by indicating the device type. For instance, you could identify your survey as an “Angioplasty device” as illustrated below:
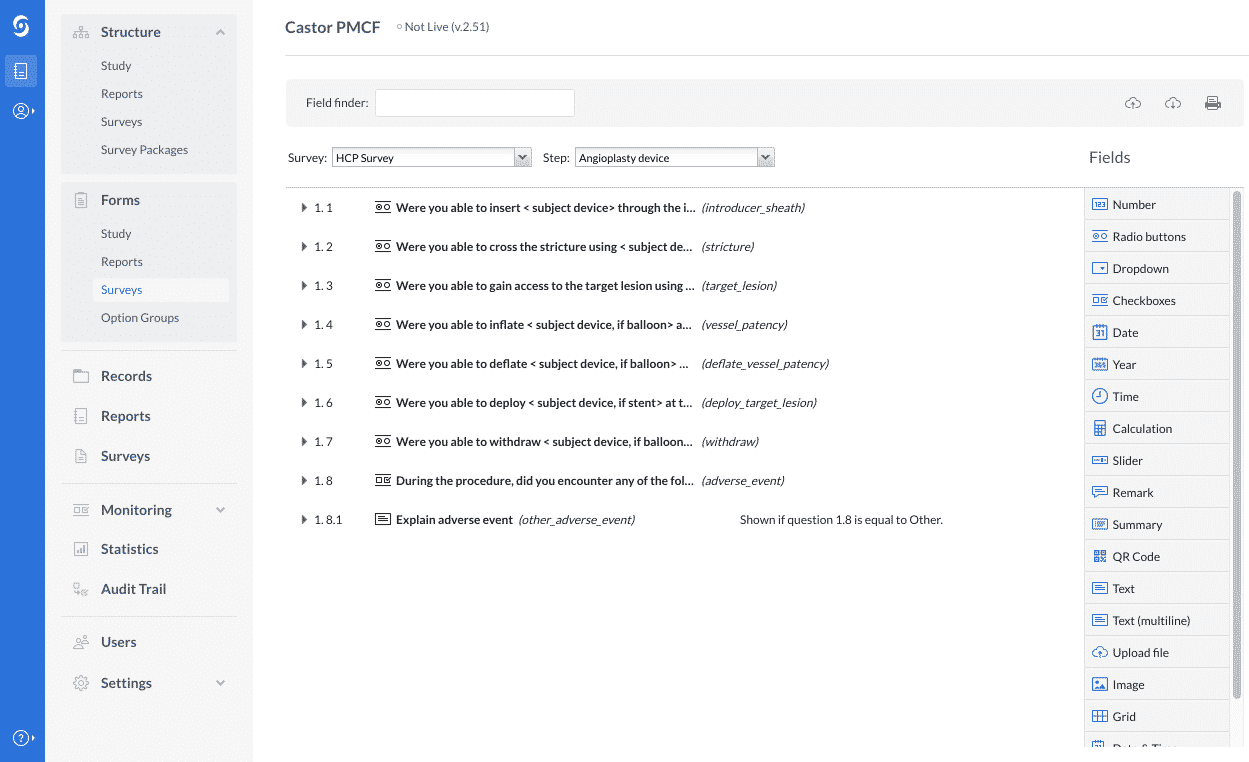
2. Second, you will type in the survey questions as in the example below:
Alternatively, you may be able to import existing survey samples from the Castor Form Exchange by downloading the survey structure and importing it into your PMCF study in Castor.
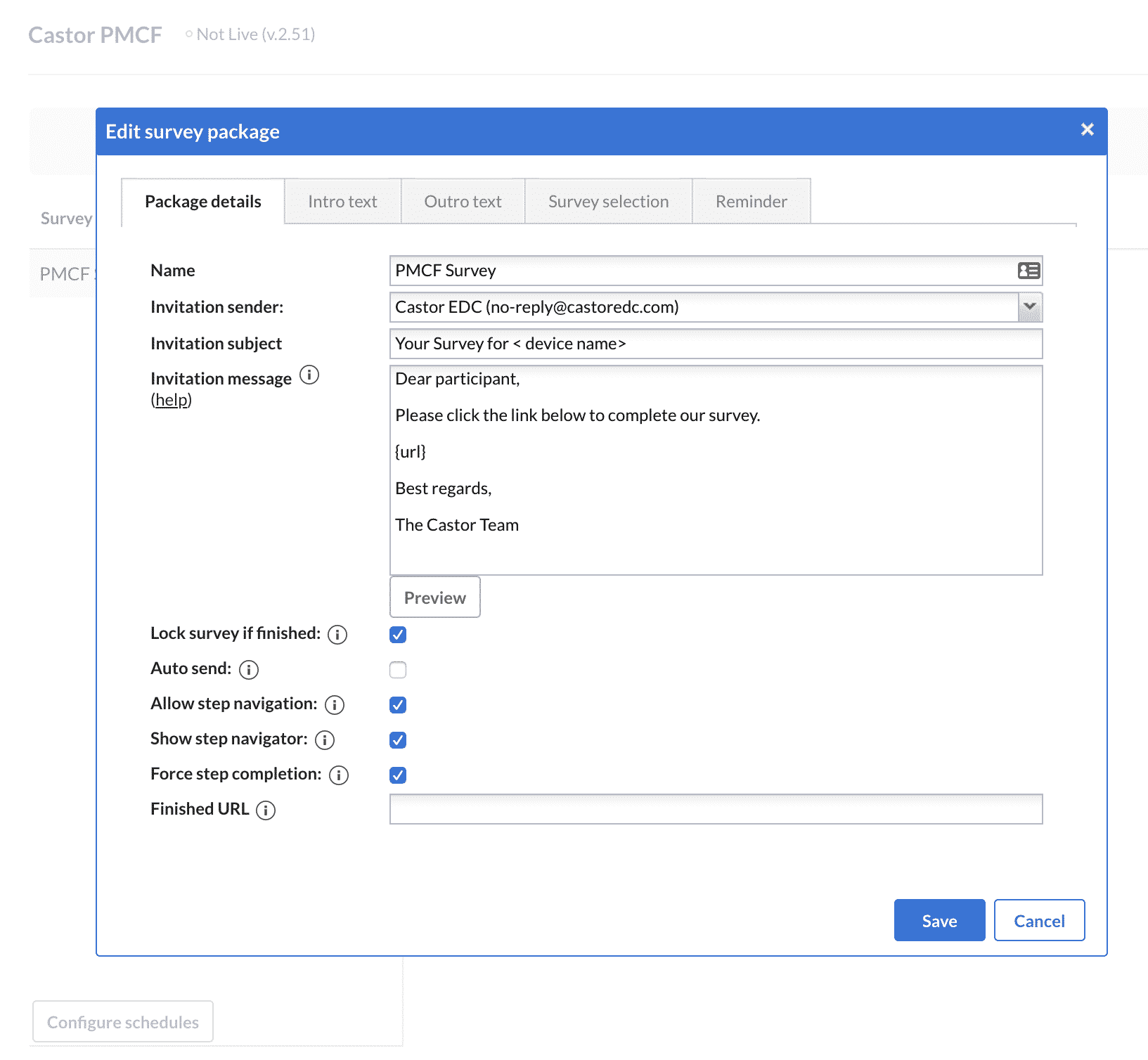
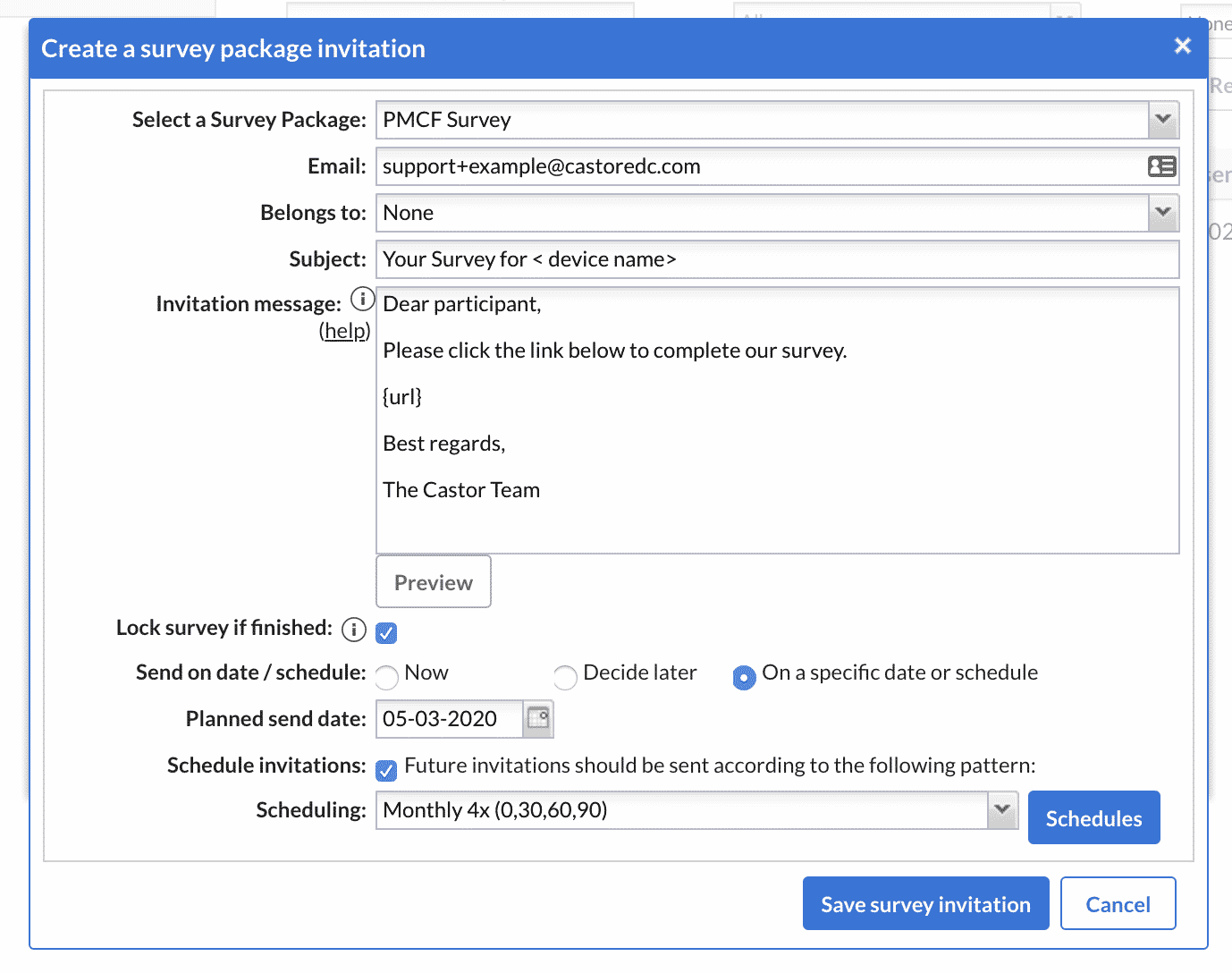
3. Once the survey structure and questions have been set up, a survey package should be created. You will be prompted to enter the sender’s name, the email subject, email text, and other survey properties as illustrated below:
4. The survey package is now ready to be sent to participants. You can send your PMCF survey to a group of participants in bulk after importing their email addresses. Alternatively, you may send your PMCF survey to single participants. Surveys can be sent on a certain date or according to a specific follow-up schedule of your choice, for instance once every month for four months as illustrated below:
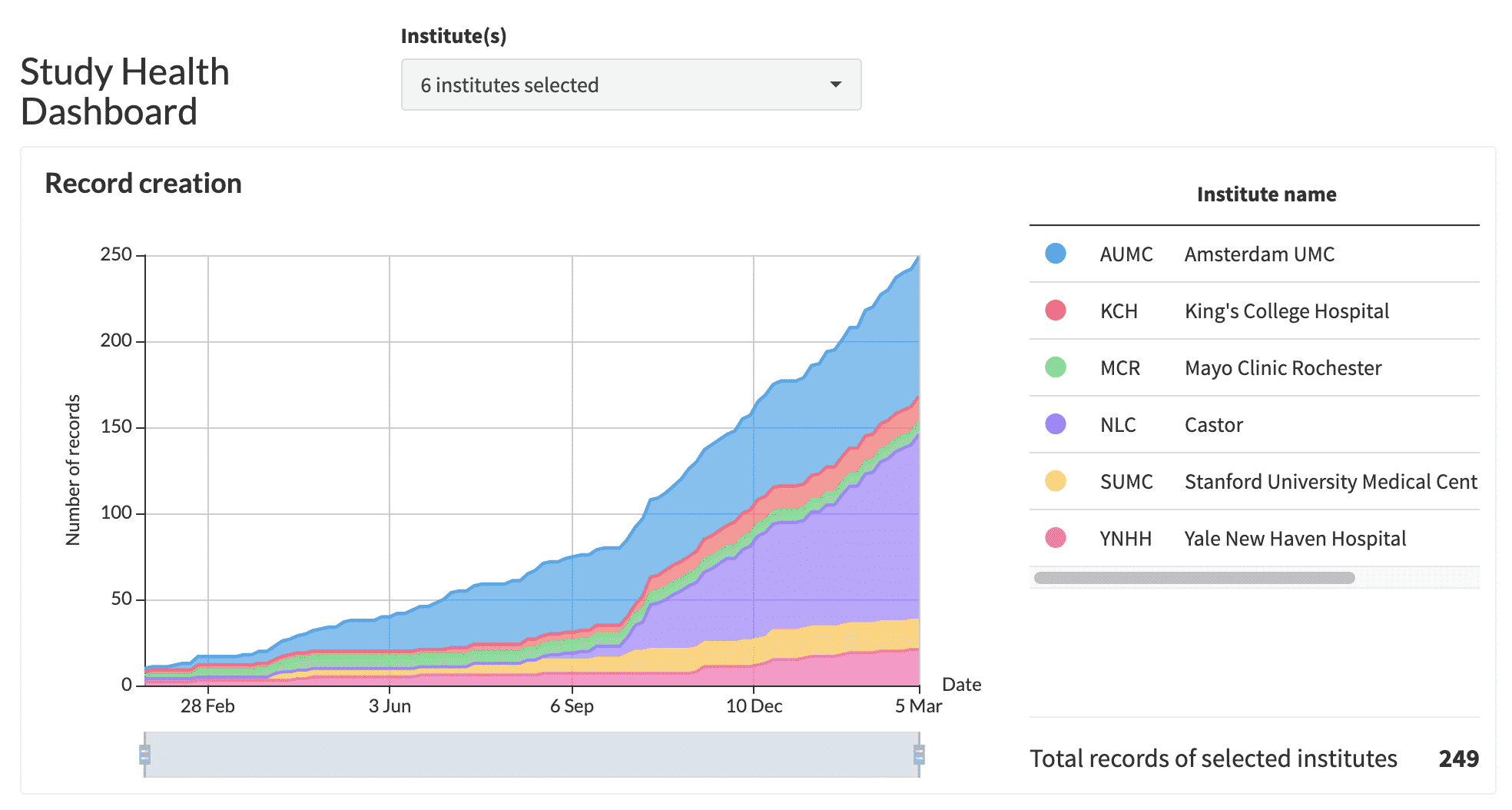
5. The respondent will receive an email with a unique link, which will redirect to the PMCF survey. PMCF data collection will begin immediately as participants start responding, and their answers will be saved in real time in your Castor PMCF study. During PMCF data collection, you will be able to track the progress of your study at any time, as illustrated below.
Study Health Dashboard showing inclusions over time:
Study Health Dashboard showing record progress: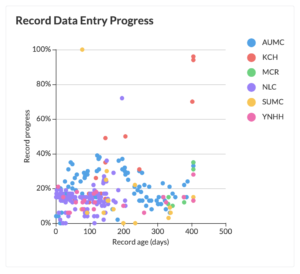
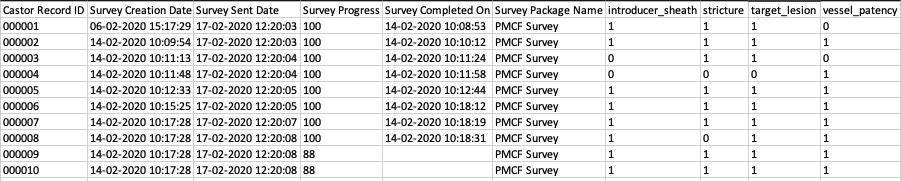
Here is an example of an Excel export:
Here is an example of a PDF export for a single survey:
Here is an overview of survey response frequency:
In conclusion, when your PMCF plans require sending surveys to physicians or questionnaires to participants, physician survey forms or electronic Patient Reported Outcome (ePRO) forms must be created to prepare for PMCF execution. Castor helps you efficiently execute your PMCF plan through ePRO and electronic Physician Surveys. Contact our team to discuss how Castor’s technology can help you fulfill MDR and PMCF data requirements.