In the 1990s, electronic data capture (EDC)—or “remote data capture” as it was then known—was quite archaic. Sometimes, it was built on a client-server architecture on a standalone device instead of an everyday PC. These systems were essentially data “silos” with no ability to easily interchange data with other systems.
Today, EDCs integrate with other types of software in the eClinical spectrum, allowing for easy data transfer. It is now commonplace to quickly and reliably import electronic data from outside sources such as imaging data sources, lab reports, and electronic health records into an EDC. At the same time, the increasing popularity of open-source EDC software and independent data standards (e.g., CDISC) are further driving productivity.
With all the buzz about the latest clinical trial tools, it can be hard to keep up with the current state of data capture. Consider this your guide to all things EDC—what it is, how researchers can use it in clinical trials, its numerous benefits, and examples of how these translate into the real world.
What is electronic data capture?
 An electronic data capture (EDC) system is software used to collect, clean, transfer, process, and store data in clinical trials. EDCs are used by contract research organizations (CROs), sponsors, and sites to conduct both simple and complex clinical trials in all phases of research. By using electronic records to capture and manage data on a digital platform, trial sponsors eliminate the need for traditional paper-based data collection. They can capture data securely and expedite the research process while ensuring data reusability.
An electronic data capture (EDC) system is software used to collect, clean, transfer, process, and store data in clinical trials. EDCs are used by contract research organizations (CROs), sponsors, and sites to conduct both simple and complex clinical trials in all phases of research. By using electronic records to capture and manage data on a digital platform, trial sponsors eliminate the need for traditional paper-based data collection. They can capture data securely and expedite the research process while ensuring data reusability.
Most EDC systems today are cloud-based so users can get secure access from anywhere. These systems collect varying types of data depending on the therapeutic area related to the clinical trial. For example, the data forms used in an oncology study might include:
- Adverse events
- Biochemistry
- Coagulation tests
- Concomitant medication
- Death information
- Demographics
- ECOG performance status
- Hematology
- Medical history
- Tumor assessments
- Treatment data
- Survival follow-up
- Urinalysis
- Vital signs
The benefits of EDCs in clinical trials
 Today, clinical trials are typically expensive and time-consuming. Worse yet, they often create a burden for their participants. Trial sponsors are challenged to optimize every aspect of the research and development process to accelerate time to market, reduce the risk of failure, and retain participants along the way—all while staying on budget.
Today, clinical trials are typically expensive and time-consuming. Worse yet, they often create a burden for their participants. Trial sponsors are challenged to optimize every aspect of the research and development process to accelerate time to market, reduce the risk of failure, and retain participants along the way—all while staying on budget.
With the help of EDCs, sponsors can build clinical trials that meet the needs of regulators, payers, and participants. By using a foundational data platform that is flexible and interoperable, researchers speed up their research while lowering costs. Here’s how.
1. Incorporate real-world data (RWD) collection
An EDC solution simplifies and streamlines data collection. Once the data collection phase is complete, statistical processing can happen quickly. An EDC supports RWD collection by:
- Ensuring that data is accurate and appropriate though inbuilt plausibility.
- Supporting remote patient participation.
- Allowing researchers to add constraints to a form to prevent inaccurate or illogical values from being entered.
- Supporting source data verification and edit checks to ensure data meets requirements for ranges and formats before it’s accepted into the trial database.
- Reducing data entry time by eliminating most paperwork.
Real-life example: COVID-RED
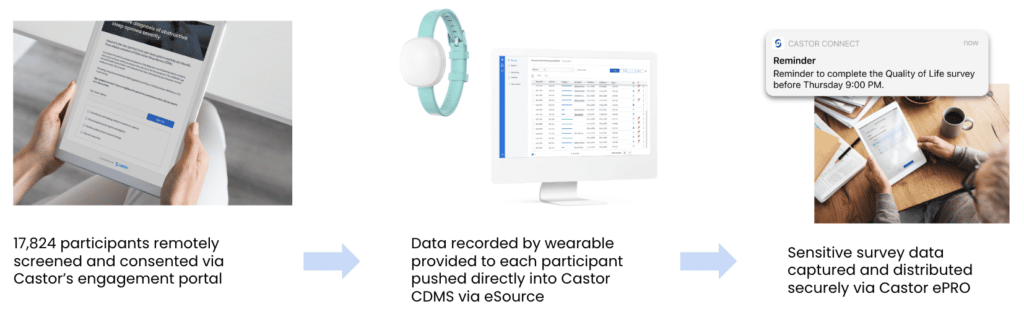
Julius Clinical’s COVID-RED (Remote Early Detection) study used wearable sensor technology and a smartphone app to measure participant vitals and provide personalized advice to get tested for COVID-19, even before symptoms appeared. The trial sponsors knew recruiting and collecting data from their 20,000-participant target would be incredibly challenging under ordinary circumstances—it would require multiple sites, complicated administrative processes, and in-person participant visits. And with 7,000 participants from a high-risk group safety during the pandemic was an issue.
Julius Clinical partnered with Castor to build an eClinical platform to run their trial remotely from start to finish. Through Castor’s clinical trial platform, over 17,800 participants were engaged and retained remotely, while data was automatically collected from thousands of medical devices and pushed into Castor EDC. According to the team at Julius Clinical: “Castor’s infrastructure ensured we were able to effectively carry out this study while keeping administrative burden, costs, and losses in participant engagement to a minimum.”
2. Directly capture global data
Clinical trials can be a global affair, with participants and researchers spread throughout the world. An EDC supports accuracy in international clinical studies through features like legible entries and automatic calculations. EDCs support global clinical trials by:
- Offering data entry and access anywhere, anytime.
- Using medical coding so that all symptoms and names of diseases are coded according to international terminology.
- Identifying and resolving data discrepancies at remote sites through digital tools.
- Allowing data managers to easily review data and quickly issue queries to sites to solve discrepancies.
- Detecting and correcting uncertain data with numerical data validation.
Real-life example: WHO Solidarity Trial
To combat the largest pandemic in recent history and generate sufficient evidence of the efficacy and safety of potential treatments, the World Health Organization (WHO) needed to mount a massive clinical trial.
The WHO needed a user-friendly system that they could deploy globally with 24/7 support. It needed to be advanced enough to support adaptive medication allocation and real-time reporting. Finally, offline data capture was a requirement due to unreliable internet in remote locations. The WHO approached Castor to help solve these challenges and quickly selected Castor as its sole EDC provider for the trial.
Castor quickly implemented a CRF in six languages, developed an adaptive randomization algorithm fitting WHO specifications, developed an offline capture tool for areas with poor telecom infrastructure, and provided 24/7 support.
“Launching and executing this ongoing trial is a remarkable achievement. In 6 months, the trial team accumulated information on the collective experience of more than 11,000 hospitalized patients in settings with varied and evolving standards of care.” – New England Journal of Medicine
3. Automate for the next generation of trial designs
Gone are the days of tedious manual data entry. Automation within clinical trials supports faster research and can speed up the return on investment of a trial. Automation within EDCs can benefit researchers by:
- Supporting remote monitoring processes (significantly reducing costs).
- Allowing market authorization to happen as quickly as possible.
- Enhancing the efficiency and productivity of a trial, further reducing the overall cost of clinical trials.
Real-life example: AusculThing
AusculThing seeks to transform the way healthcare providers detect heart murmurs by enabling them with a far more precise tool than the human ear. To train the algorithm to work more effectively, they would need to collect an enormous amount of audio and diagnosis data across 5 different sites.
The AusculThing team turned to Castor’s eSource platform, a system that would enable them to collect, capture, and process a significant amount of patient data from several sources, and feed it directly into Castor’s EDC. By the end of the trial, researchers had collected audio data recorded by a digital stethoscope and additional diagnosis info from over 1,700 patients – all without the need for manual source data verification. With data that’s healthy, integrated, and accessible through one interface, AusculThing can now train its AI algorithm much more efficiently and make considerable strides in creating an auscultation system that’s more accurate and efficient for healthcare systems worldwide.

4. Streamline collaboration workflows
Throughout studies, trial teams, monitors, and auditors need to capture, process, and access data from anywhere, at any time. EDCs allow data can be quickly shared with relevant personnel, allowing for a seamless exchange of information by:
- Supporting real-time data access while limiting the time that has to be spent on query management.
- Allowing authorized personnel to access, download, and print status reports at any point.
- Supporting decision-making and adaptive trial designs with easy access to data insights.
- Offering search options so users can easily find and filter the exact information that they need.
- Storing everything in one central location that provides greater visibility.
- Linking data collected in eCRF from one form to another for analysis.
- Requesting only the data needed for a particular patient’s circumstances at a specific time.
Real-life example: RSP Systems—Using Monitoring to Track Study Progress
Over the past few years, RSP Systems has worked to bring their GlucoBeam monitor to market as a non-invasive and convenient glucose monitoring technology for diabetes patients. To do so, they’ve conducted multiple small clinical trials testing various settings on the device to improve protocols, estimate the signal-to-noise ratio, and increase the accuracy of the measurements. But the success of their studies depended on gathering highly accurate data ready for analysis. And with multiple trials running—some multicenter—they needed a bird’s eye view of all their studies with minimal manual effort.
Using Castor EDC, RSP Systems can now seamlessly capture clinician, patient, device, and any other external data and get a complete overview of all the data linked to an individual patient. In particular, they’ve made great use of the monitoring feature in Castor EDC for a comprehensive overview of all the queries, data validations, and dropped verifications in each of their studies. They could find all the active validation fields in their study forms, reports, or surveys and easily filter by the type—Exclusion, Warning, and Message. From there, they could find out from which site the validation errors originated, compare the field values with the reference values defined during the validation setup, and jump to the step with the validation errors.
5. Manage mid-study changes with ease
EDC systems are flexible and adjustable, helping to meet the needs of the study as they change throughout the process. They support mid-study changes by:
- Allowing feedback from study monitors and participants right away.
- Enabling protocol amendments midway through the study.
- Providing instant eCRF updates across all sites of a multi-site (or even multi-continent) study.
- Leveraging data traceability and access controls.
- Using designated permissions with most actions carried out by specific roles, further ensuring data security.
Real-life example: AKRN Scientific Consulting
AKRN Scientific Consulting joined forces with a medical device manufacturer to innovate a way to keep a donor heart oxygenated in transport to its recipient. Together, they designed a clinical study to test their transportation solution. They then partnered with Castor to help design a clinical research ecosystem that would keep up with the evolving needs of the study.
As the randomized clinical trial expanded into several sites in different countries, study managers wanted to incorporate feedback from various study monitors and participants while still accommodating the varied regulatory and clinical needs. The protocol needed to be a living document that could evolve, reflecting the advice and parameters of each site. Fortunately, Castor’s eCRF made this possible, allowing changes to be quickly programmed and automatically uploaded to each site. As a result, each site always had the most up-to-date version of the eCRF.
What features should you look for in an EDC?
While purchasing a robust EDC may seem like a significant investment, the money that it saves, in the long run, makes it well worth the expense. Here’s what to look for:
- A user-friendly interface that supports collaboration between multiple sites and researchers globally.
- The option of auto checks and data limits to reduce errors.
- Guaranteed compliance with privacy and data protection regulations.
- Seamless integration with existing tools (e.g., wearables or legacy systems).
- The ability to reuse data collected from the trial for future use.
- Technical advisors to help set up and offer guidance at every stage.
- An eCRF designer with options for reusable templates and built-in edits checks.
- Both auto-generated and manual queries.
- Built-in metrics reporting to giant insights throughout the study.
How EDCs are Part of a Paradigm Shift within Clinical Research
Currently, clinical trials use isolated technology tools—such as eCRFs—to collect, manage, and analyze data. eCRFs are study forms built into EDCs that let researchers and clinical staff enter data directly into the system. These digital, typically web-based questionnaires ensure data compliance with privacy, security, and Good Clinical Practice regulations. Researchers can tailor eCRFs to fit each study, saving time and money.
While eCRFs may seem progressive compared to paper-based data entry, they represent a siloed system where data is entered and processed separately. They are a holdover on the way to a seamless, single platform. So what’s the broader solution?
The clinical trial industry is shifting from siloed, cumbersome processes to fully connected, interoperable clinical trial platforms for collecting and analyzing data. To do so, the trial tech community is laying the groundwork for a new way of running clinical trials: pairing an EDC with fully decentralized clinical trial (DCT) technology. By fully leveraging automation and interoperability, this innovative paradigm puts all data management components into a single system.
The CDMS/DCT combination incorporates all clinical trial data components, from electronic consent (eConsent) forms to scheduling, televisits, and data from devices, consumer wearables, and patient apps. CDMS/DCT has the potential to import real-world data (RWD), label electronic source (eSource) data, and conduct automated edit checks to ensure the quality of the data. An API architecture setup allows data collection from almost any source at lightning speed. The goal is simple: a single system to collect, store, and process all clinical trial data.
Despite all the flux within the clinical trial industry, one thing is sure: therapy breakthroughs happen faster and more often when researchers have access to affordable, user-friendly, and compliant data solutions. The good news? In just a few clicks, researchers can build a study with Castor’s data platform—without any prior technical knowledge. Better yet, they can grow alongside Castor as they step into the future of fully integrated trials.



