When the COVID-19 pandemic caused global disruption to everyday life, site visits for clinical research became difficult or even impossible. Immediately, decentralized clinical trials (DCT) became essential to maintaining research continuity. In the recent past, this approach to trials was viewed as new and innovative, but the pandemic has necessitated DCT as a mainstream clinical research strategy.
During the pandemic, Castor actually saw increased access to clinical trials—including medical device trials—made possible through the development and implementation of decentralized methods and/or remote technology, such as eConsent and telehealth. At Castor, we anticipate that 70% of clinical protocols will incorporate some element of decentralization in the future. Experts project that moving forward, at least one in five appointments with a healthcare provider will be conducted via telemedicine.
Our team firmly believes that DCT is great for both patients and the industry: applied correctly, incorporation of decentralized methods increases recruitment and retention, offers a personalized participant experience, and reduces the administrative burden on sites and study staff. In this post, we will look at how EDC, eConsent, ePRO, and APIs enable researchers to easily conduct decentralized and hybrid clinical trials.
How to leverage remote technology in a medical device trial
Most commonly, decentralized does not mean purely siteless trials—it usually involves a hybrid approach where limited site visits are paired with remote patient communication. DCT brings researchers into homes through mobile technologies (e.g. phone apps) and connected data capturing systems like wearable devices. For this reason, the Castor eClinical platform was designed to capture data from a huge variety of modalities.
Almost any medical device study protocol can incorporate elements of DCT. Here’s how:
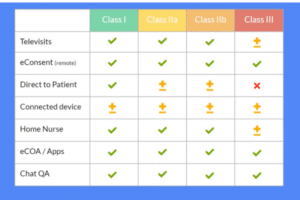
Televisits
Through video interaction with patients and online assessments, televisits can eliminate the need for on-site clinical assessments. For Class I to II b devices, it is usually possible to conduct all visits through televisits. Class III devices will almost always be bound to sites for the initial implementation or surgery that involves the device, but it may be possible to do follow-up visits remotely.
eConsent
Fully remote eConsent can be incorporated into the protocol for any device classification so long as IRB/ethics board requirements are met. From the comfort of their home, participants can complete both online and offline questionnaires in a form that offers them a choice and preference of web, mobile or native app—from eligibility criteria to screening questions to the enrollment portal. Finally, an investigator from a centralized site walks the participant through the consent process via video call.
Direct-to-patient
Where permissible, medical devices can be shipped directly to patients. This is usually feasible for lower-risk devices such as Class II a and sometimes Class II b—depending on the particulars of the device. Of course, a Class III device is typically not something that can be shipped to a participant.
Connected devices
Connected devices are able to continuously monitor the patient and collect data. Prioritize selection of devices that are user-friendly and easy for the participant to set up at home. The use of commercial fitness wearables can accomplish this, but the validation and precision of such devices should be examined closely. These can be used to monitor activity levels, step count, heart rate, pulse oximetry, and more. Other common health data sources, such as spirometry, can also be incorporated during televisits.
Home health nursing
By sending a home health nurse to collect data from the participant’s home, the need to travel to hospitals for sample collection is reduced or eliminated. Depending on the type of device or data to be collected, there may be several activities that require the professional oversight and skills of a home health nurse.
ePRO and eCOA
Electronic Patient-Reported Outcomes (ePRO) and Electronic Clinical Outcome Assessment (eCOA) can be used in almost every study. This is often a simple matter of questionnaires being done online or via an app instead of on paper. Importantly, with the right platform ePROs can be scheduled or triggered by advanced rules or automation workflows, eliminating the need to contact participants individually.
Chat and video calling
The power of video brings human connection into a clinical trial. Rather than conducting an in-person follow-up visit on-site, follow-ups can be done online through video conferencing. Importantly, video can aid with identity verification. It can also be used to assess if patients are using a device correctly: Is the blood pressure cuff on properly? Are the body sensors in the right spots? Finally, video can be used for coaching purposes, such as during an at-home spirometry test.
All said DCT is a great fit for most device classes—even implantables! DCT elements are an amazing fit for most device trials since connected devices can be shipped to patients and push data to a cloud platform. DCT also removes the need for source data verification in some cases. When a device pushes data directly to the trial’s master data file, it eliminates the need for SDV since there isn’t any room for human error. Lastly, devices can be shipped with a serial number, thereby creating traceability—an important aspect of a clinical trial audit trail.
M2M in Clinical Trials
Clinical trial systems need to be able to integrate with other systems and exchange information. Machine to machine, or M2M focuses on leveraging devices that are connected directly to the Internet of Things (IoT). Many other industries with strict regulatory requirements—such as transportation and energy—are already using M2M technology in a secure manner. In some cases, clinical researchers can ship pre-configured, connected devices to patients which can then push data directly into a Cloud platform—all without participants having to do much setup.
The benefits of M2M go beyond quicker onboarding, however, and include:
- Increased compliance and adherence
- Lower incidence of technical issues and support requests
- Increased access to clinical trials and research opportunities
- 2G data available worldwide and over longer distances
One way Castor supports M2M within clinical trials is through device integrations. We’re always looking to partner with other companies to facilitate the direct transmission of device data to Castor’s backend. Depending on the type of device, data is directly pushed to the platform through a dedicated connection either when the participant uses the device or the participant chooses to record a measurement in their patient portal.
Castor is device agnostic and can be connected to almost any device through SIM cards, Bluetooth, or oAUTH (where patients give our platform permission to read their wearable data through the wearable cloud environment). Medical device researchers leverage this capability to continuously capture device data during device development studies or monitor patients remotely during a clinical trial.
Castor’s API is designed to let device developers connect devices directly to Castor. In fact, the open, transparent, and easy-to-code Castor API is one of the most-used components of Castor. We continue to invest in its development with a vast roadmap of upcoming features, many based on user requests.
CASE STUDY: COVID-REDWhat do DCTs look like in the real world? When nine organizations from four countries united to assess whether a wearable device could predict the onset of COVID-19, they knew they needed a unique protocol to make it work. Naming the project COVID-RED (Remote Early Detection), they enlisted Castor’s help, who provided a remote-friendly platform on which the trial could run. This novel trial called for thousands of participants to be enrolled through an automated portal. Then the Netherlands national press picked up on the project and ran the story, resulting in a sudden surge of participants. Fortunately, the trial was set up to be scalable and handle huge amounts of data. In the end, around 20K individuals enrolled—all without a single site visit. As the study progressed, participants were directly shipped a pre-activated device, the AVA wristband. Finally, they filled out ePRO questionnaires throughout the duration of the trial. The entire trial was fully decentralized and proved to be an exceptional solution during a challenging time. |
The benefits of decentralized methods for medical device trials
Modern medicine needs to be patient-centric, and this includes clinical trials. Rather than merely focusing on data for submission, more researchers are recognizing that making trials patient-centric results in higher quality data and better participant retention.
Fortunately, adopting decentralized methods can help optimize the patient experience. A hybrid or even siteless approach means less travel or no travel for site visits and lower time investment for participants. By adopting decentralized methods—such as reducing the need to travel to sites, adopting eConsent, and hiring site staff with language and cultural experience—clinical trials can be made more inclusive of a disease’s true population distribution. This is a huge win for both patients and medical device companies.
Overall, clinical research is undergoing a paradigm shift. There is greater acceptance of using direct-to-patient methodologies and more recognition of the value of leveraging remote technology to support decentralized protocols. At Castor, we hope to see DCT extend into all trials as we work alongside researchers in the pursuit of the ultimate goal of democratized clinical research.
The future of clinical trials is here via DCT. Will you be part of it? Click here to find out how you can use Castor’s eClinical platform in your next medical device trial.