Clinical trials are experiencing significant disruption worldwide due to the COVID-19 pandemic. Medical research is not immune to the effects of physical distancing, strained healthcare systems, and an emotionally volatile environment affecting trial participants. Clinical trial teams are facing regulatory changes, participant safety issues, funding issues, and a tech crunch as they flip from in-person to remote trials.
The Impact
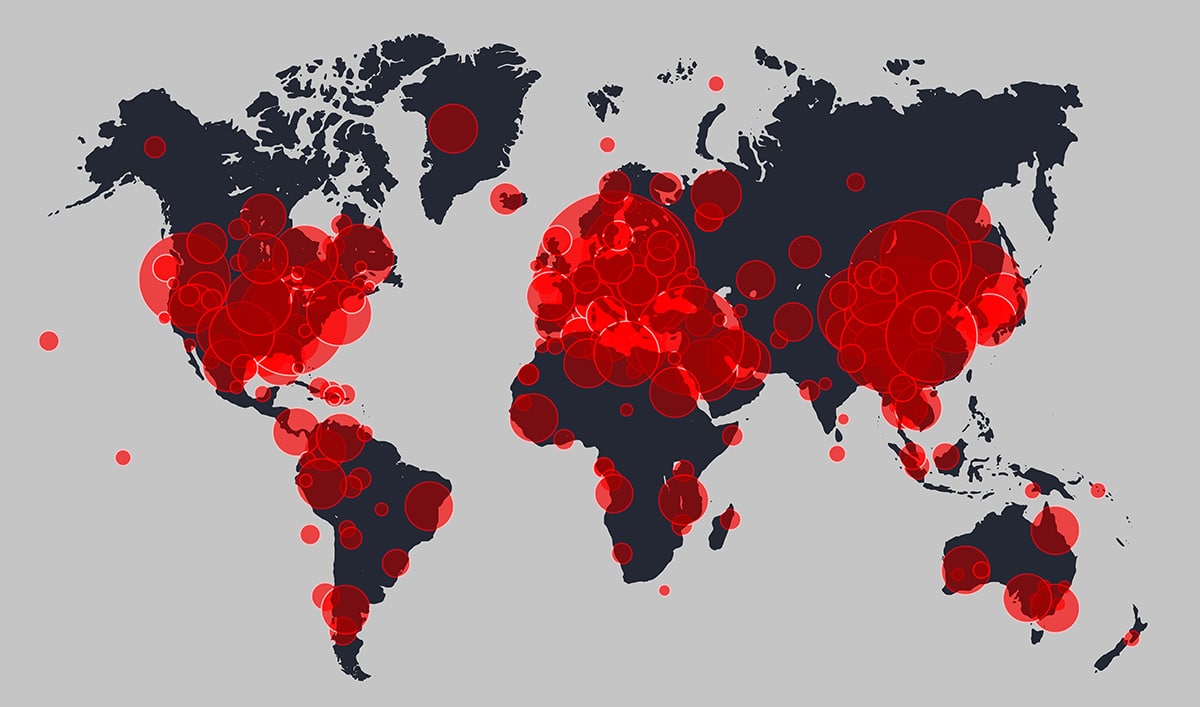
As of this publication, there are 262,366 recruiting clinical trials globally which may be affected. The full impact in Europe, America, and elsewhere remains to be seen. So far, we know that there has been a slow response to “flatten the curve” and epidemiologists are already warning of a fresh spike of cases in the fall. Realistically, we can expect that clinical studies may be significantly disrupted for at least 12 months.
Additionally, COVID-19 is consuming healthcare resources for both treatment of the ill and trials. Study sponsors will likely be forced to prioritize between COVID-19 and in-progress clinical trials. Existing trials may be slowed down or even stopped, especially where trial sites are located in hospitals. A number of EU sites, such as Poland, France, Italy, and UK, have suspended trial activities with the exception of treating ongoing patients to prevent missed treatments. Trial participants in many areas are not allowed to travel to clinic sites or are uncomfortable doing so.
Currently, the biggest challenge in the EU is the lack of site staff availability to do anything trial-related as many are being re-deployed to Emergency Rooms. Other sites are not allowing Clinical Research Assistant (CRAs) on site, putting current trials at risk of shutdown unless they quickly find a remote strategy. And, of course, due to quarantine and social distancing orders in effect almost all clinical trials are suffering from slowed or stopped enrollment of participants. Trials are also grappling with delayed investigational product shipments.
As we observe China slowly get back to business, we will be looking closely at their clinical trial progress for clues about the future of other research.
The Initial Response
As a first step, it’s essential to limit exposure risk to research participants, clinicians, and study coordinators. Investigators also need to pivot how they are conducting studies and may need to adjust protocols.
Protect Participants
Now’s the time to differentiate between what visits are essential to be done in-person and what can be done remotely. Minimizing exposure for study participants may require a reduction of in-person appointments and any other circumstances that would require the participant to engage in non-essential travel. As we’ll discuss further on, this may involve using local labs or monitoring patients from home.
Some patients involved in medical device trials may be chronically or critically ill and at risk of harm if the trial is paused without a contingency plan. Consider arranging private transportation for the participant to come to the trial site, if it is open, and the participant would otherwise take public transportation. And if a patient simply doesn’t feel comfortable participating in a trial right now, their wishes must be respected.
Protect Staff
Protecting staff may mean restricting non-essential staff from being on-site. If this is the case, drastic work-from-home measures will be needed. As leaders are no doubt aware, this requires a tremendous amount of change management as individual workers respond differently to change depending on their personalities. There is also the need to establish alternate ways to engage all team members, whether it’s through daily huddle, connecting via video, installing Slack channels, etc. Regardless of the challenges, research leaders must step up to manage their teams from afar.
Regulatory Guidance
During this time of upheaval, it’s important to review what regulations have been changed or loosened to accommodate trials impacted by COVID-19.
- FDA Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Pandemic (18/3/2020)
- EMA: Guidance on the Management of Clinical Trials during the COVID-19 (Coronavirus) pandemic (20/3/2020)
- MHRA: Advice for Management of Clinical trials in relation to Coronavirus (12/3/2020)
“The regulatory guidance, from my perspective, offers a lot more flexibility than we’ve seen before,” says Baljit Samra, Castor Advisory Board member and former COO of the Duke Clinical Research Institute. “I’d certainly encourage you to read both the FDA and EMA guidance in particular.”
After reviewing the material and deciding what protocol changes might be necessary, try to fast-track the process as much as possible to keep your study moving forward. There may be a need to report to the institutional Review Boards (IRBs) and Regulatory Bodies with whatever changes you make. Admittedly, it can be hard to pinpoint right now where changes can be made without explicit approval, so we recommend that you follow the guidance from these regulatory bodies to ensure that you don’t make any changes to your trial that will not be allowed.
Doubtlessly, notified bodies will consider deviations from business-as-usual when they are considering clinical trial results. However, the onus remains squarely on investigators to record protocol modifications, deviations and missing data. In order to meet quality and compliance challenges, document reasons for data and the impact of delays. Record stoppage, reduction or modified inspections and/or audits. Capture risk logs for COVID-19 with CROs and partners.
“Whatever approach you take within the boundaries of the regulatory guidance, I would document it so that you are in a position to really justify your approach,” advises Baljit.
Conduct Trial Risk Assessments
Each and every trial is different and only its researchers will know that trial intimately. Regulators are therefore asking researchers to conduct detailed risk assessments, giving priority to the safety of trial participants. When doing so, factor in the many disruptive aspects caused by COVID-19 and prepare for inevitable deviations to processes and to protocols.
The first big question: Is my trial regulated by FDA, EMA or any other regulatory bodies? The more tightly-regulated the trial, the fewer protocol changes that you’ll be able to make without explicit approval. On the other hand, if a study is being conducted in an academic setting or it is an investigator-initiated study, there may be more options.
While conducting a risk assessment, be sure to review the risk to participants. If the trial itself is beneficial to the health of participants, then traditionally the trial would need to continue so as not to endanger patients’ health. This might be true of oncology studies or rare disease studies, where the patients may derive great benefit from continuing treatment. Conversely, if visiting the hospital site would be detrimental to a patient’s health, that might be a point where you stop enrollment. In short, take a common sense and ethical approach when deciding whether your trial should continue.
Simplify Protocol
When considering how to proceed with a trial, divide clinical data into three categories:
- Must-have and needs to be collected on-site
- Must-have but can be collected remotely
- Not needed
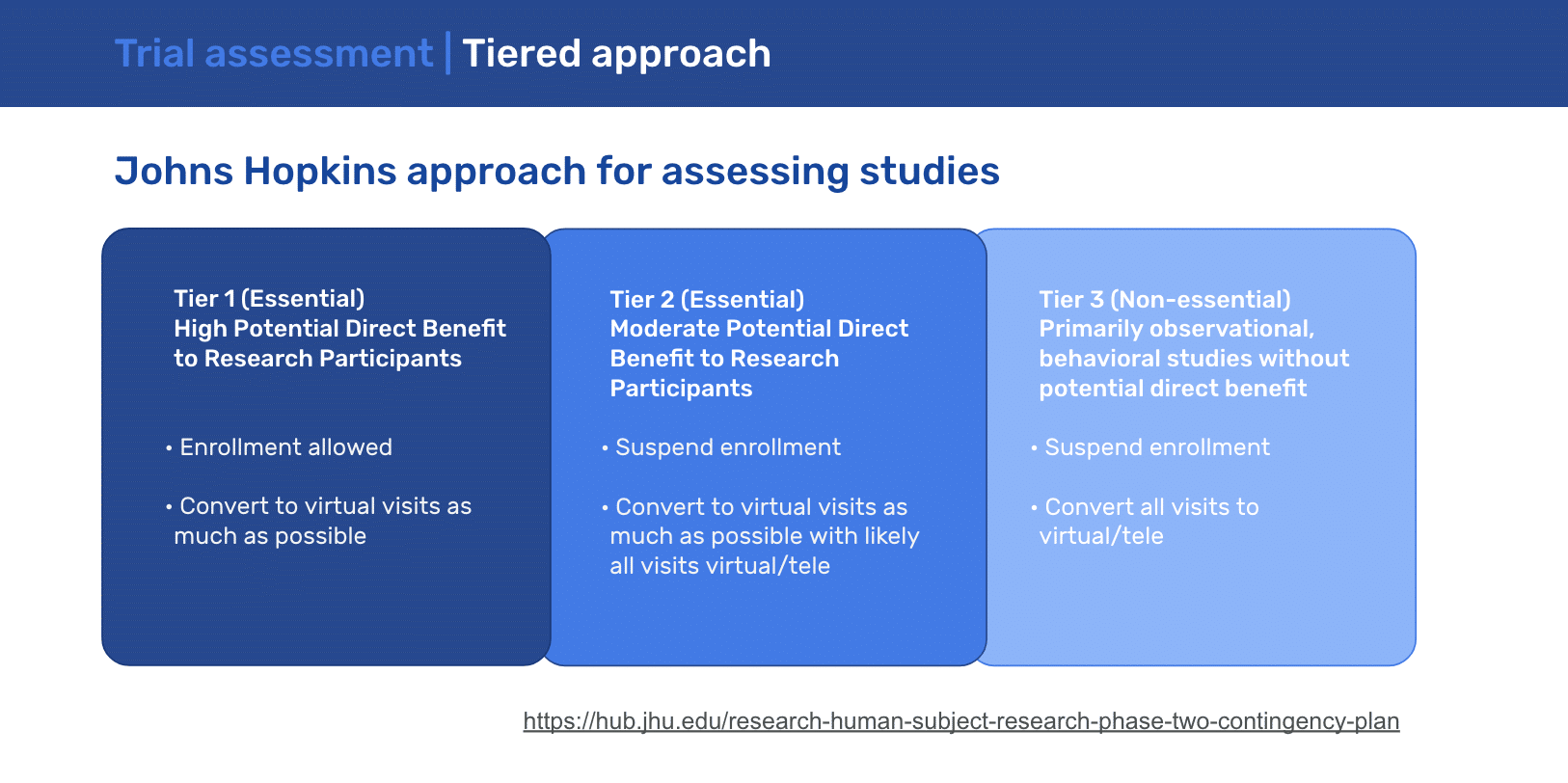
Now is the time for protocol simplification and a focus on collecting essential data. In deciding how specific clinical studies should be handled during this pandemic, John Hopkins advised the following guidance:
Leverage Technology to Modify a Study
Potential modifications may be possible to keep a trial alive through the pandemic. Look at your entire protocol and think about where you can start to plug in technology. Dip deep and get really creative by looking at both in-the-box and out-of-the-box options.
Before implementing any new tech solutions, review the original trial consent form—did you explicitly state which technology you’d be using? If so, that would significantly restrict your flexibility because the patient consented to a very particular approach. Most consent forms that we see, however, do not have such restrictions and would allow you for flexibility in introducing new technology.
If you have access to a data protection office or legal department in your company or at your institute, try to involve them early. For example, if you are thinking about adding videoconferencing or a new document management solution or any new technology platform, get the experts involved right away to go through the approval process. And if you work at a big company or an academic institution, collaborate with others so you only need to work with a single vendor and only go through one approval process to get the tech you all need.
Activate remote enrollment
The biggest question here is whether decentralized enrollment is possible: Can you enroll patients without them actually visiting the site? Over 85% of researchers expect moderate to significant impact on recruiting new study participants, according to a recent survey by Castor, so if that’s a possibility, you may be able to continue enrollment. This might involve a video call where you go through the consent form with the participant, followed by the participant filling out an e-consent form. Stay as close as possible to the original process. Regulatory bodies will consider alternative consent options, but they will still require approval. Contact our team to learn how we can support implementation of remote enrollment.
Mail Equipment to Participants
There are a lot of measurements typically done on-site that could potentially be done remotely. Enable virtual clinical trial data collection by mailing user-friendly diagnostic devices to your participants such as pulse-oximeters, spirometers, glucose meters, etc. If there’s a large enough budget, consider switching to IOT devices which can provide plug-and-play in-home measurements. Look at endpoints and assess whether there is a device that you could ship to the patient which would be plug-and-play where they can start sending these measurements to an EDC system such as Castor EDC.
Use Existing Tech Tools
Based on your local regulations, you may be able to use mainstream options like Confrere or similar platforms to connect with patients. For example, video calls can be a great way to do assessments while observing the patient. Look into what secure messaging tools are available through telehealth platforms. Be sure to incorporate the use of these tech tools into your protocols to promote patient engagement and collect data. Look for certifications such as HIPAA, HITRUST, ISO-270001, SOC-2, NEN-7510, and GDPR compliance. Go for brands that are already operating in healthcare because they will likely already have necessary certifications in place.
Leverage Local Resources
Consider the use of local facilities or laboratories for study visit procedures where the principal investigator is in agreement and the patient is in agreement. Even in places with emergency measures in place, specimen collection centers are likely considered essential services and may remain open.
Issue Lab Slips Remotely
Prepare lab slips for blood draws and urine specimens remotely so that a participant does not need to enter a clinic in order to get the lab slip made. This may be done through e-mail, fax, or other means. The patient can go directly to the specimen collection centre and skip a clinic visit.
Engage with Participants
Set up a content feed or blog to keep subjects up to date on study status. Engage with trial participants by:
- sending regular updates via email
- using ePRO software for additional measures outside of protocol
- using social media for engagement (while respecting anonymity)
- using video calls to stay in touch
“Historically, I’ve always seen that our industry tends to be a more conservative industry,” says Baljit. “As long as we are conscious of the regulatory requirement around privacy and security, at this point in time, it’s going to be a blank sheet of paper as far as how we address research needs in both a technological and virtual manner.”
Remote Monitoring
Nearly 50% of researchers surveyed by Castor say they will be looking for technology to support them in remote source data validation and remote monitoring, so consider what tools are needed to ensure monitors are effective while working remotely. Processes must remain compliant with regulations (e.g. no unencrypted PDFs with personal data being exchanged), and researchers should carefully assess whether they are using compliant technology for this purpose, such as HIPAA-compliant screen-sharing technologies. A round-up of recommendations from Castor Advisory Board member Craig Lipset can be found here.
Our Role
Our Castor team has been busy surveying our clients’ needs. From our conversations, we know that what clinical trials need right now is technology allowing investigators to observe participants, instruct them to complete assessments, and record their observations directly into an eSource form. In a nutshell, they need technology to support data collection as it moves from an onsite environment into a virtual one. Learn how Castor supports research continuity here.
As part of our effort to support COVID-19 studies across the world, we released a global surveillance and research platform with the following capabilities:
-
- Online enrollment portal for patients
- eConsent module
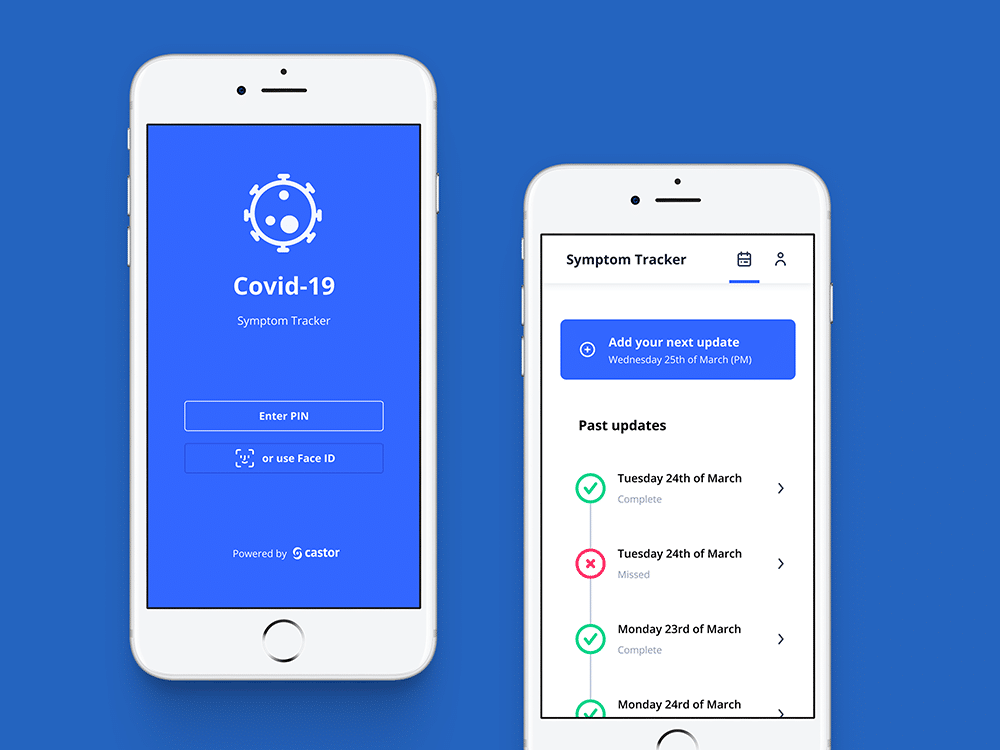
- Symptom Tracker app
- Real-time reporting across the enrolled population
The above are all integrated into Castor’s Electronic Data Capture (EDC) system and are available for use with all trials. We are also working to launch other remote-friendly features as quickly as possible. As a company, we are intently interested in supporting our clients to keep their studies running and our entire team is working hard to support those efforts. Now is the time to implement robust strategies to mitigate any issues with ongoing studies as a result of the COVID-19 crisis.
Reach out with any questions or concerns—we’re ready to help!