In September 2020, Castor CEO Derk Arts, MD, Ph.D., led a webinar on electronic informed consent (eConsent) challenges and opportunities. The webinar included a lively question and answer session focused on critical issues for decentralized and hybrid clinical trials requiring electronic consent forms. It distilled the more prominent concerns for members of the research community, particularly concerning COVID-19 and the pressing need to implement digital technologies in clinical trials.
Here, we’ve pulled together the most popular questions in a broader discussion of eConsent to share the concerns of leading research scientists and how eConsent provides an effective solution.
Question 1: How does eConsent align with the traditional consent process?
In brief, eConsent is the electronic version of informed consent. Valid informed consent for research must include three major elements:
- Disclosure of information
- Competency of the participant (or surrogate) to make a decision
- Voluntary nature of the decision
US federal regulations require a full, detailed explanation of the study and its potential risks. See the US Food and Drug Administration (FDA) guidance for complete details. Meanwhile, let’s look at how Castor eConsent addresses these required elements.
Disclosure of information
With Castor eConsent, investigators can disclose trial information with more interactive and visual methods, such as diagrams, images, videos, and screen readers for accessibility. These tools typically increase participant engagement and comprehension.
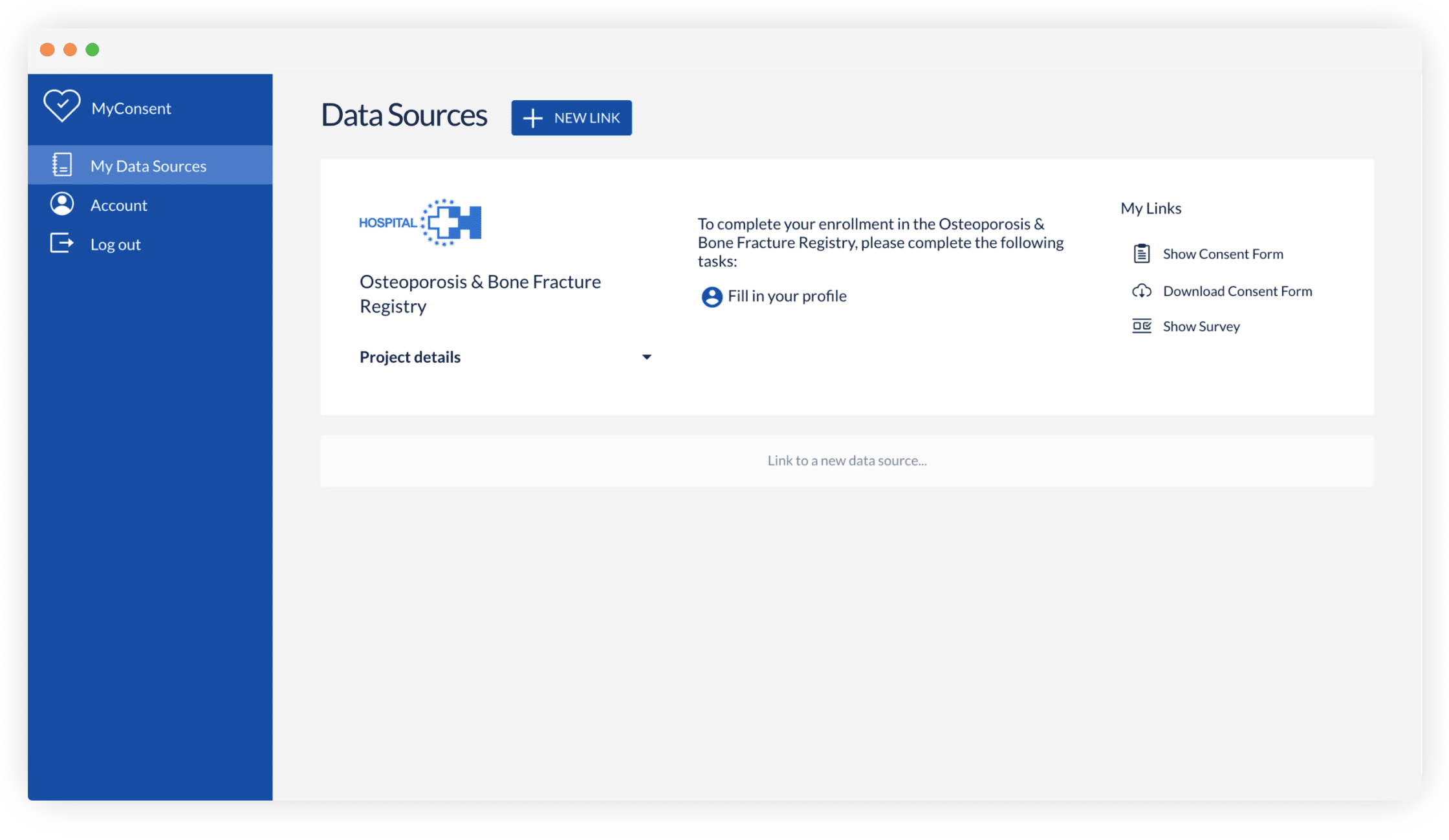
In addition, with Castor, critical consent information is always available online for the participant to refer to. Immediate access to these details can attract participants, increase their confidence, and contribute to better patient retention.
Competency of the participant (or surrogate) to make a decision
Castor eConsent’s video conferencing capability provides investigators who perform the consent calls with a real-time, visual interaction with participants. As a result, investigators can determine firsthand if participants are competent to make informed decisions based on direct observation of their behaviors.
Voluntary nature of the decision
As with competency, investigators can determine if participants are making voluntary decisions to engage in clinical trials by observing their behaviors on the video calls. For example, an investigator can readily detect if a participant competently and willingly coordinates the call session and attends—a positive indication of a voluntary decision to participate in the trial.
Castor eConsent video conferencing is a critical component.
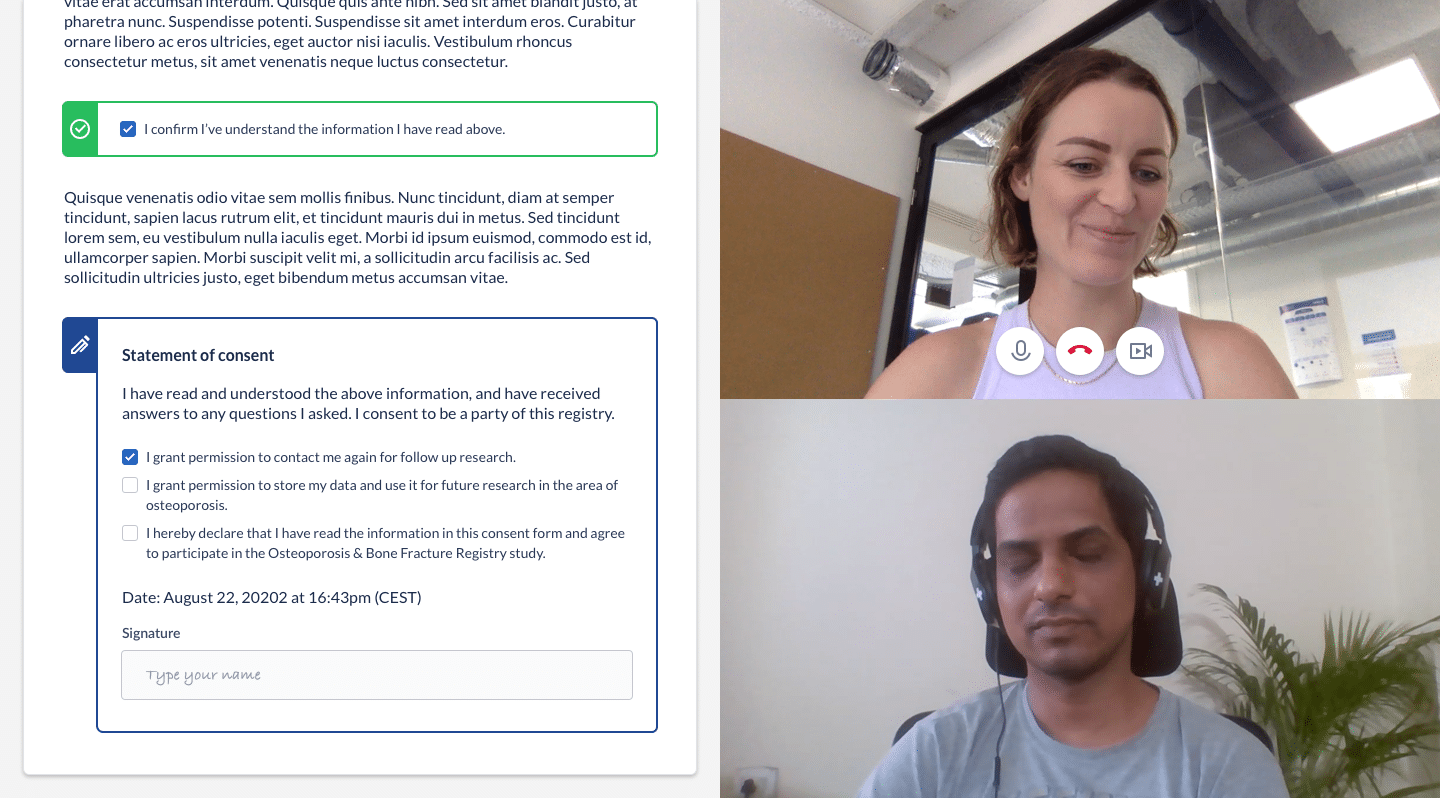
Castor’s unique built-in video conferencing capability is a powerful solution that connects participants with site staff with the added layer of visibility. With video call capability, researchers can resolve questions in real-time; visually verify signatures and identification; thoroughly engage participants in the consent process; and meet all FDA requirements.
Question 2: How can an eConsent solution support re-consenting?
eConsent significantly simplifies re-consenting, providing a much more streamlined process than traditional informed consent. To better understand re-consent scenarios, let’s review the different consent types and how eConsent improves them.
Types of consent
- Traditional consent refers to a participant providing consent for a research study in person with paper informed consent forms (ICFs). eConsent makes this process less burdensome on an administrative level and removes the requirement for site visits.
- Continued consent refers to obtaining consent repeatedly from a study participant even after obtaining initial consent at the study’s start. eConsent automates repeat consents, so researchers can electronically update the ICF and remotely perform re-consenting.
- Broad consent is a specific type of consent that focuses on the storage, maintenance, and secondary research uses of both identifiable private information and identifiable biospecimens. The Final Rule governs broad consent. eConsent makes broad consent more traceable by providing an electronic trail.
How would re-consent work in case of updates to the ICF?
The ICF contains the required information and can require updates during a study for various reasons, including accommodating continued or broad consent. When the ICF is updated, you must re-consent participants.
With eConsent, re-consenting is simple and straightforward. Upon approval from the Institutional Review Board (IRB) or ethics committee, researchers implement necessary updates to the information in the enrollment portal, schedule another video call, and let the participant give or decline consent.
Also, you can add eConsent to existing studies if the IRB allows for it.
Question 3: How is eConsent adoption advancing globally?
An electronic signature is always integral to eConsent, but eSignature requirements vary by country, impacting eConsent adoption. If the study requires IRB approval, researchers must fully understand eSignature needs for each country in which they operate. While not every country accepts both eConsent and eSignature, the situation is evolving (along with necessary pandemic measures), and it is anticipated that eSignature acceptance will become universal.
Access Castor’s guide to eConsent regulations in the US, Europe, and Israel to learn more about country requirements and how you can best prepare.
The importance of understanding local IRB guidelines
In general, the IRB and ethics committee requirements for paper and electronic consent are the same:
- IRB-approved language and format
- Must contain all elements of informed consent required by HHS or FDA
- Signature(s) unless waived by IRB
- Study staff involvement
- Copy provided to the participant
Countries and regions differ in policy, so it is essential to understand local IRB guidelines.
Using eConsent in a country that does not allow electronic signature
In countries that do not accept eSignature, Castor’s research indicates that a participant can often sign a paper form while on the video call and then mail in that form. Although this method entails paperwork, you retain two key benefits: the eClinical platform tracks the consent status, and participants can access trial information online at any time. Sponsors and contract research organizations (CROs) should validate that their local IRB will approve this workflow.
Question 4: How does eConsent contribute to participant enrollment, recruitment, and retention?
eConsent not only streamlines and accelerates patient enrollment and recruitment for researchers but also enhances the participant experience, providing such benefits as
- The ability to consent from any location
- Use of mobile apps on their own devices
- Responsive mobile design
- Integration of interactive media
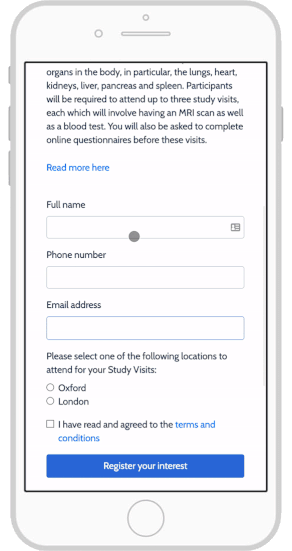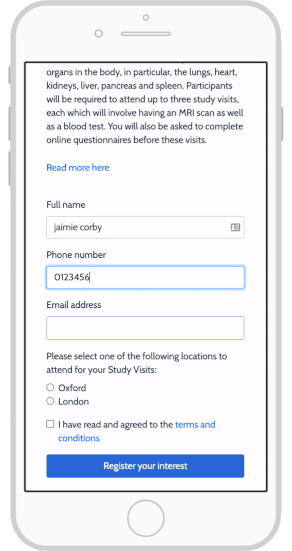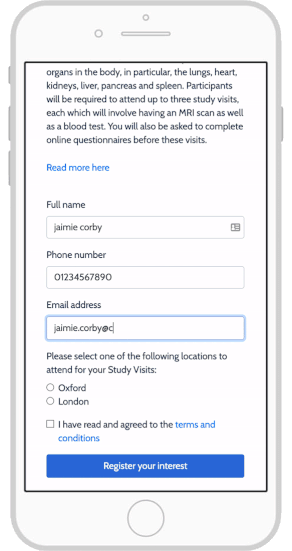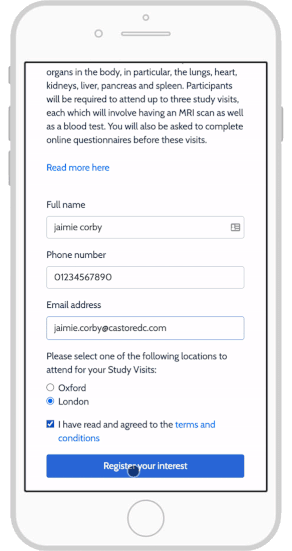
Together, these features help participants better understand the trial’s goals while also setting appropriate expectations, and create a more trusting relationship with the study team. By facilitating more accessible participant enrollment and retention and improved participant compliance, eConsent can directly translate into improved study outcomes.
Castor eConsent provides a digital consent solution that provides all these benefits with full compliance and flexible features. With it, you can configure a custom landing page for your specific trial needs. For example, this page can let participants select a proximate site, thus aligning patient location with site location—a particularly useful feature for studies that require an occasional site visit.
What next?
Do you have other questions about eConsent and decentralized and hybrid clinical trial technology? Schedule a demo and start modernizing your ICF workflow today.