Our recent webinar featuring PRO expert Ari Gnanasakthy, RTI Health Solutions, and Derk Arts, CEO & Founder, Castor, sheds light on the complexities and advancements in measuring PROs, drug tolerability, and quality of life in cancer trials. Here, we distill the insights shared and explore the implications for future research.
Cancer studies are not what they used to be
 Derk and Ari kicked off their conversation by discussing how cancer research is getting more and more complex and with this ever-evolving landscape, a focus on patient-reported outcomes (PROs) and clinical outcome assessments (COAs) has never been more critical. With the emergence of innovative treatments such as plasma-derived therapies, CAR-T therapies, reductions in chemotherapy cycles, and a surge in novel oral medications, the shift towards more personalized and targeted therapies underscores the necessity to move beyond traditional PRO methodologies.
Derk and Ari kicked off their conversation by discussing how cancer research is getting more and more complex and with this ever-evolving landscape, a focus on patient-reported outcomes (PROs) and clinical outcome assessments (COAs) has never been more critical. With the emergence of innovative treatments such as plasma-derived therapies, CAR-T therapies, reductions in chemotherapy cycles, and a surge in novel oral medications, the shift towards more personalized and targeted therapies underscores the necessity to move beyond traditional PRO methodologies.
In this new era, a more patient-centric approach is needed, ensuring that treatment strategies not only combat the disease effectively but also offer real-time insights into treatment efficacy, patient well-being, tolerability, and quality of life (QoL).
“Let’s stop talking about patient reported outcomes, instead let’s talk about the patient experience and the ways we measure QoL within all the nuances of cancer trials.“
– Ari Gnanasakthy, RTI Health Solutions
Navigating the regulatory landscape
The webinar delved into the changing regulatory landscape for cancer trials, particularly for measuring PROs. As treatments like CAR-T cell therapy become more prevalent, capturing the authentic patient journey has become more crucial for understanding both efficacy and safety. Regulatory bodies are increasingly emphasizing the importance of real-world evidence, pushing for a more comprehensive view of treatment impacts. This shift necessitates meticulous planning in data collection, not only to meet regulatory approval requirements but also to address the downstream needs of payers. Having this dual focus ensures that the collected information serves both immediate regulatory purposes and future payer evaluations, facilitating a smoother transition from clinical approval to market access. With the rise of personalized and targeted therapies, the reliance on outdated PRO methodologies is no longer viable. Clinical trial design needs to reflect a balance between scientific rigor and patient-centricity, ensuring research outcomes are both scientifically valid and meaningful to patients’ lives.
Who says the drug is tolerable?
The Patient. A critical aspect of the discussion between Ari and Derk centered on the concept of measuring treatment tolerability and QoL within cancer research. The conversation highlighted a need to shift perspectives, with emphasis on understanding how tolerability influences patient adherence and overall treatment outcomes. Ari emphasized the importance of viewing tolerability through a comprehensive lens and throughout treatment cycles.
“Tolerability is not something that can be measured at the beginning of each cycle when patients are reasonably healthy. Tolerability is something that needs to be measured when patients are having issues and when the drug is at peak, during cycles…probably on a weekly basis.”
– Ari Gnanasakthy, RTI Health Solutions
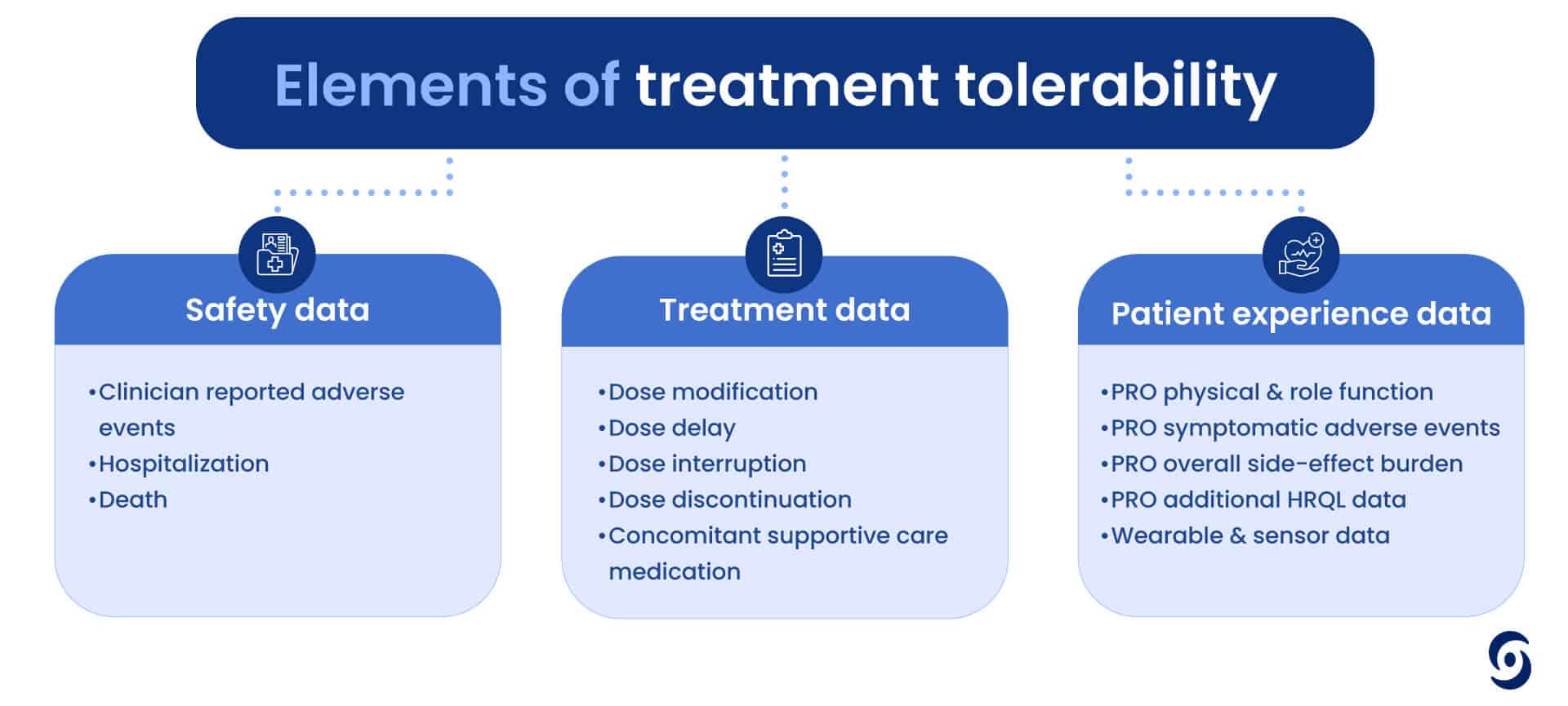
But how do we consistently measure something so subjective in a scientific way? Tolerability is not just one measure but a number of elements that need to be considered, including safety data, treatment data and PROs (like physical and role function, side effect burden or even hair loss). It is clear we need to consider both the physical and psychological nuances of patient experiences when assessing treatment tolerability.
 What does the future hold for ePRO?
What does the future hold for ePRO?
Finally, Derk and Ari spent time looking ahead at how the role of technology and ePRO in cancer research is expected to expand. Wearables, BYOD (Bring Your Own Device) strategies and advanced scheduling for data collection will play pivotal roles, enabling real world data collection that reflects the true impact of cancer treatment on patients’ lives.
This digital-first approach is anticipated to improve data quality and drive more patient engagement. By engaging patients through their own smartphones and leveraging technology they are familiar with, clinical trials can more accurately measure the true patient experience, thereby improving participation and adherence. Additionally, ePRO technology offers solutions to challenges around data integrity and overcoming the “parking lot effect,” where responses may be influenced by the immediate environment of data collection. This evolution marks a pivotal step towards more patient-centric research and ultimately better outcomes.
“It’s now clear that we’re dealing with three layers of data. Initially, we have the core data that has been the focus for the past two decades. Added to this are scheduled QoL and tolerability data. The final layer encompasses wearable and ad hoc data, enriching our understanding of tolerability. This stratified approach presents a significant opportunity to enhance patient outcomes.”
– Derk Arts, Castor Founder & CEO
And, from the sponsor side, it’s imperative to consider the influence of Quality of Life (QoL) and tolerability measures on the drug’s value proposition. For sponsors, overlooking this aspect could lead to a significant gap in their value proposition.
The single line item from the FACT-G questionnaire, GP5, which asks patients if they are “bothered by side effects of treatment,” has demonstrated a strong predictive value for both patient dropout and disease progression when patients report being troubled by side effects. This underscores the importance of integrating patient-centric measures into clinical trial design and evaluation to enhance patient retention and treatment outcomes.
Getting started with Castor eCOA / ePRO
To deepen the understanding of eCOA / ePRO technology for sites and sponsors, and to ensure fast study builds, Castor welcomes study teams to explore our eCOA / ePRO platform by offering early access, and allowing them to personally navigate the patient experience. Our comprehensive onboarding process and dedicated customer support play a pivotal role in this commitment. Additionally, we provide the option to work with synthetic data upfront, offering a proactive approach to risk mitigation.
At the heart of our platform is a patient-centric approach, designed to significantly improve the trial experience for participants. By prioritizing the patient perspective, we aim to enhance engagement and collect valuable real-world data. This data is crucial for gaining insights into treatment effectiveness, patient well-being, tolerability, and quality of life, in the ever changing landscape of cancer research.
For more information, watch our on-demand webinar:
eCOA / ePRO and Cancer Research – Measuring the true patient experience
Follow us on LinkedIn: Castor
Learn more about Castor eCOA / ePRO


