This is the first of three case studies covered in a webinar Castor held with AKRN Scientific Consulting. Watch the webinar for more insights from the AKRN team. You can also download this case study below.
Heart transplants have always had a limited shelf life. A retrieved heart has an incredibly short amount of time to make it from a donor to a recipient – in most cases, only 4 to 6 hours.1
A medical device manufacturer, who we’ll call Company X, had an innovative idea: what if we perfused the heart (that is, kept it oxygenated) while moving it from the donor to the recipient? They developed a solution that fed oxygenated blood to the heart as it was in transit and awaiting a donor, dramatically lengthening the time a heart transplant could remain viable outside the body.
Early premarket tests with animal hearts suggest this innovative solution may be able to extend the heart transplant period from 4 hours to 24 hours – just imagine how far and wide the pool of donors can expand, and how many more lives can be saved, with 20 hours longer to transport a donor heart.
Designing a complex clinical study that can incorporate real-time feedback
Success in extending the transplant period with animal hearts inspired Company X to join forces with AKRN Scientific Consulting, a contract research organization (CRO) based in Madrid, Spain, to conduct a clinical study. Their primary goal would be to answer the question, “Will non-ischemic heart preservation prolong organ function in an adult heart transplant patient?” But designing a clinical study to test this was complex: the study would need to incorporate real-time feedback while maintaining consistent protocol amendments. Protocol amendments can reduce cycle time, difficulties recruiting study volunteers, and protocol design complexities, but they’re incredibly costly and time consuming for researchers. Implementing a single protocol amendment can take more than 2 months, and cost more than $453,000.2 It was critical that AKRN had a clinical trial platform that was quick to adapt and easy to scale as the study changed – an electronic data capture (EDC) system would be essential to ensuring that real-time modifications could be made to the research protocol with ease, and that regulatory needs were met at each site globally.
Expanding clinical trial feedback to multiple sites with a living document
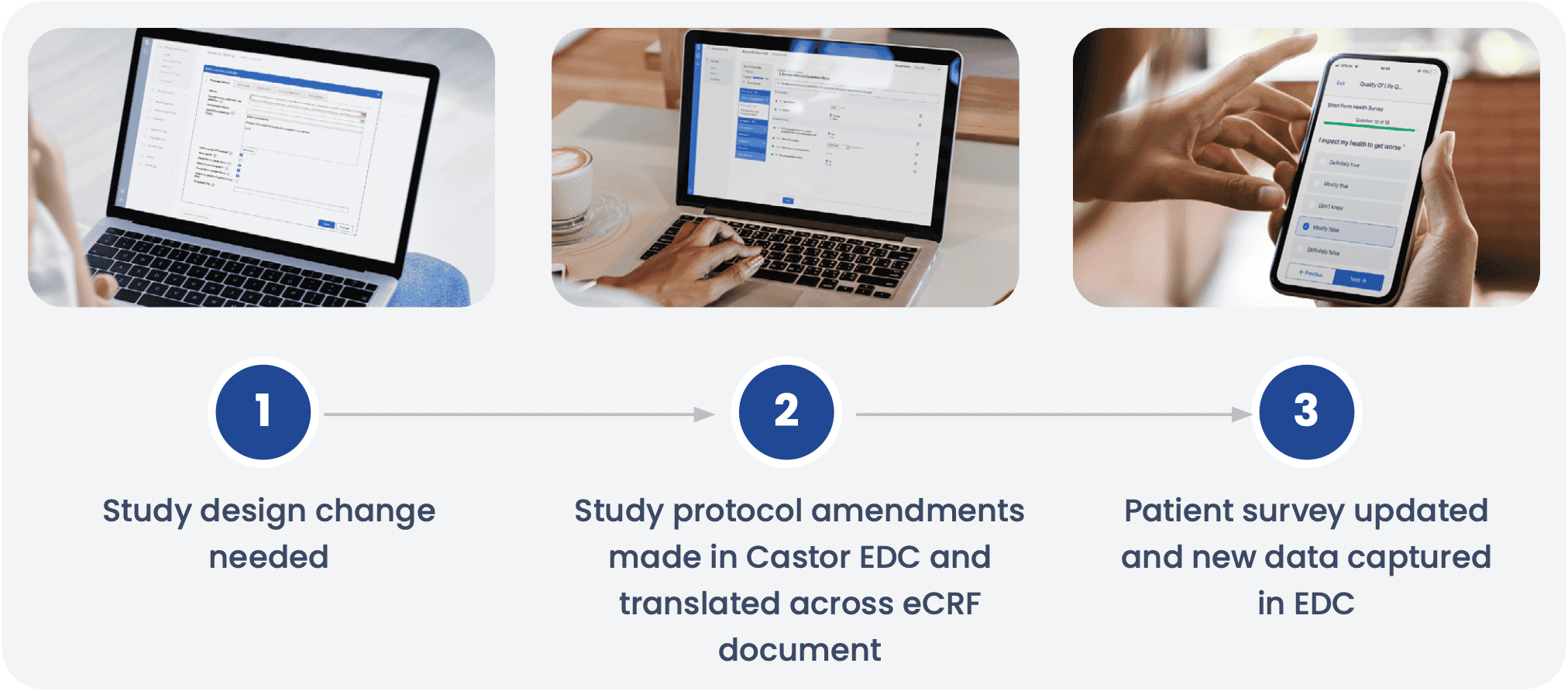
 AKRN Scientific Consulting partnered with Castor to help design a clinical research ecosystem that would keep up with the evolving needs of the study. As the randomized clinical trial expanded into several sites in different countries, study managers wanted to incorporate the feedback they heard from the various study monitors and participants and continue to accommodate the varied regulatory and clinical needs. The team needed a living document for the protocol to evolve and reflect the advice and parameters of each site. Castor’s electronic case report form (eCRF) made a living document possible, allowing for quick changes to be programmed, and automatically uploaded to each site.
AKRN Scientific Consulting partnered with Castor to help design a clinical research ecosystem that would keep up with the evolving needs of the study. As the randomized clinical trial expanded into several sites in different countries, study managers wanted to incorporate the feedback they heard from the various study monitors and participants and continue to accommodate the varied regulatory and clinical needs. The team needed a living document for the protocol to evolve and reflect the advice and parameters of each site. Castor’s electronic case report form (eCRF) made a living document possible, allowing for quick changes to be programmed, and automatically uploaded to each site.
Using a flexible eCRF to make protocol amendments with ease
Accommodating new clinical sites and new regulatory needs in each country also meant that real-time modifications would need to be made to AKRN’s reaserch protocol. Each clinical trial center responded with feedback on the trial and made suggestions, many of which were incorporated.
What could have been an arduous and painstaking task was simplified with Castor’s eCRF. With programmers ready to incorporate changes into the eCRF, the protocol changes were made and propagated to all the sites instantly. As a result, each center in the clinical trial always had the most up-to-date version of the eCRF.
“Castor is flexible enough to be used by beginners, and powerful enough for experienced clinical operations professionals.”
– Iñigo Marquet De Solís, Clinical Research Scientist & Data Manager, AKRN By Namsa
Overcoming trial complexities and developing life-saving treatments

AKRN’s study has more than 200 participants, and with the ability to collect data and feedback from sites in real-time and amend their protocol accordingly, is quickly moving toward completion. Despite the complexity of this study and ongoing COVID-19 travel restrictions, Castor’s EDC proved both easy to use and robust.
“The Castor EDC is flexible enough to be used by beginners,” said Iñigo Marquet de Solís, Clinical Research Scientist & Data Manager, AKRN by NAMSA, “and powerful enough for experienced clinical operations professionals.”
Thanks to this innovative solution and the ability to test it in a clinical setting, a reality where human hearts can survive outside the human body for up to 24 hours, and patients who desperately need a donor heart have a far greater chance of receiving one, is within reach.
References
- “Matching Donors and Recipients – OrganDonor.gov.” 20 Apr. 2021, https://www.organdonor.gov/learn/process/matching. Accessed 24 Aug. 2022.
- “Protocol Amendments: a Costly Solution – Applied Clinical Trials.” 30 Apr. 2011, https://www.appliedclinicaltrialsonline. com/view/protocol-amendments-costly-solution. Accessed 24 Aug. 2022.